are organic or inorganic lipids?
Hi, welcome to solsarin site, in this post we want to talk about“are organic or inorganic lipids”,
stay with us.
are organic or inorganic lipids?
A lipid is any of various organic compounds that are insoluble in water. They include fats, waxes, oils, hormones, and certain components of membranes and function as energy-storage molecules and chemical messengers.
lipid
lipid, any of a diverse group of organic compounds including fats, oils, hormones, and certain components of membranes that are grouped together because they do not interact appreciably with water. One type of lipid, the triglycerides, is sequestered as fat in adipose cells, which serve as the energy-storage depot for organisms and also provide thermal insulation. Some lipids such as steroid hormones serve as chemical messengers between cells, tissues, and organs, and others communicate signals between biochemical systems within a single cell.
The membranes of cells and organelles (structures within cells) are microscopically thin structures formed from two layers of phospholipids molecules. Membranes function to separate individual cells from their environments and to compartmentalize the cell interior into structures that carry out special functions. So important is this compartmentalizing function that membranes, and the lipids that form them, must have been essential to the origin of life itself.
Water is the biological milieu—the substance that makes life possible—and almost all the molecular components of living cells, whether they be found in animals, plants, or microorganisms, are soluble in water. Molecules such as proteins, nucleic acids, and carbohydrates have an affinity for water and are called hydrophilic (“water-loving”). Lipids, however, are hydrophobic (“water-fearing”). Some lipids are amphipathic—part of their structure is hydrophilic and another part, usually a larger section, is hydrophobic.

lipid
Amphipathic lipids exhibit a unique behaviour in water: they spontaneously form ordered molecular aggregates, with their hydrophilic ends on the outside, in contact with the water, and their hydrophobic parts on the inside, shielded from the water. This property is key to their role as the fundamental components of cellular and organelle membranes.
Although biological lipids are not large macromolecular polymers (e.g., proteins, nucleic acids, and polysaccharides), many are formed by the chemical linking of several small constituent molecules. Many of these molecular building blocks are similar, or homologous, in structure. The homologies allow lipids to be classified into a few major groups: fatty acids, fatty acid derivatives, cholesterol and its derivatives, and lipoproteins. This article covers the major groups and explains how these molecules function as energy-storage molecules, chemical messengers, and structural components of cells.
Have you ever tried to put oil in water?
They don’t mix. Oil is a type of lipid. Lipids are molecules such as fats, oils, and waxes. The most common lipids in your diet are probably fats and oils. Fats are solid at room temperature, whereas oils are fluid. Animals use fats for long-term energy storage and to keep warm. Plants use oils for long-term energy storage. When preparing food, we often use animal fats, such as butter, or plant oils, such as olive oil or canola oil. There are many more type of lipids that are important to life. One of the most important are the phospholipids that make up the protective outer membrane of all cells (Figure below). These lipid membranes are impermeable to most water soluble compounds.
Organic Compounds
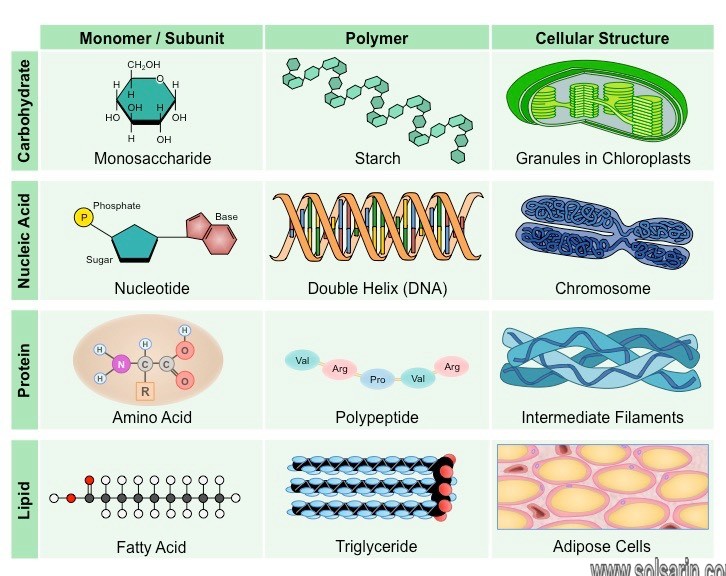
Carbohydrates
Almost all organisms use carbohydrates as sources of energy. In addition, some carbohydrates serve as structural materials. Carbohydrates are molecules composed of carbon, hydrogen, and oxygen; the ratio of hydrogen atoms to oxygen and carbon atoms is 2:1.
Simple carbohydrates, commonly referred to as sugars, can be monosaccharides if they are composed of single molecules, or disaccharides if they are composed of two molecules. The most important monosaccharide is glucose, a carbohydrate with the molecular formula C6H12O6. Moreover, Glucose is the basic form of fuel in living things. In multicellular organisms, it is soluble and is transported by body fluids to all cells, where it is metabolized to release its energy. Glucose is the starting material for cellular respiration, and it is the main product of photosynthesis .
Three important disaccharides are also found in living things: maltose, sucrose, and lactose. Maltose is a combination of two glucose units covalently linked. The table sugar sucrose is formed by linking glucose to another monosaccharide called fructose.
|
What is an Organic Compound?
|
Compounds that occur naturally only in the bodies and products of organisms and contain carbon and hydrogen.
|

Main Classes of Carbon Compounds
There are four principle groups of organic compounds that contribute to much of the structure and function of a cell
Carbohydrates
- Most abundant organic compound found in nature, composed primarily of C,H and O atoms in a common ratio – (CH2O)n
- Principally function as a source of energy (and as a short-term energy storage option)
- Also important as a recognition molecule (e.g. glycoproteins) and as a structural component (part of DNA / RNA)
Lipids
- Non-polar, hydrophobic molecules which may come in a variety of forms (simple, complex or derived)
- Lipids serve as a major component of cell membranes (phospholipids and cholesterol)
- They may be utilised as a long-term energy storage molecule (fats and oils)
- Also may function as a signalling molecule (steroids)
Nucleic Acids
- Genetic material of all cells and determines the inherited features of an organism
- DNA functions as a master code for protein assembly, while RNA plays an active role in the manufacturing of proteins
Proteins
- Make over 50% of the dry weight of cells; are composed of C, H, O and N atoms (some may include S)
- Major regulatory molecules involved in catalysis (all enzymes are proteins)
- May also function as structural molecules or play a role in cellular signalling (transduction pathways)
Fatty Acids
The common feature of these lipids is that they are all esters of moderate to long chain fatty acids. Acid or base-catalyzed hydrolysis yields the component fatty acid, some examples of which are given in the following table, together with the alcohol component of the lipid. These long-chain carboxylic acids are generally referred to by their common names, which in most cases reflect their sources. Natural fatty acids may be saturated or unsaturated, and as the following data indicate, the saturated acids have higher melting points than unsaturated acids of corresponding size. The double bonds in the unsaturated compounds listed on the right are all cis (or Z).
The higher melting points of the saturated fatty acids reflect the uniform rod-like shape of their molecules.Moreover, The cis-double bond(s) in the unsaturated fatty acids introduce a kink in their shape, which makes it more difficult to pack their molecules together in a stable repeating array or crystalline lattice. The trans-double bond isomer of oleic acid, known as elaidic acid, has a linear shape and a melting point of 45 ºC (32 ºC higher than its cis isomer). The shapes of stearic and oleic acids are displayed in the models below.


Fatty Acids
Two polyunsaturated fatty acids, linoleic and linolenic, are designated “essential” because their absence in the human diet has been associated with health problems, such as scaley skin, stunted growth and increased dehydration. These acids are also precursors to the prostaglandins, a family of physiologically potent lipids present in minute amounts in most body tissues.
Because of their enhanced acidity, carboxylic acids react with bases to form ionic salts, as shown in the following equations. In the case of alkali metal hydroxides and simple amines (or ammonia) the resulting salts have pronounced ionic character and are usually soluble in water. Heavy metals such as silver, mercury and lead form salts having more covalent character (3rd example), and the water solubility is reduced, especially for acids composed of four or more carbon atoms.
| RCO2H | + | NaHCO3 |     |
RCO2(–) Na(+) + CO2 + H2O |
| RCO2H | + | (CH3)3N: |     |
RCO2(–) (CH3)3NH(+) |
| RCO2H | + | AgOH |     |
RCO2δ(–) Agδ(+) + H2O |
Steroids
Steroids are found in animals within something called hormones. Moreover, The basis of a steroid molecule is a four-ring structure: one ring with five carbons and three rings with six carbons. You may have heard of steroids in the news. Moreover, Many bodybuilders and athletes have used anabolic steroids to build muscle mass. The steroids make their bodies add more muscle than they would normally be able to. Those anabolic steroids help bodybuilders wind up stronger and bulkier (but not faster). Steroids are also used in necessary medicines. Some help people with acne, while others are used as muscle relaxers for injuries.
Never take drugs to enhance your body. Those athletes are actually hurting their bodies. They can’t see it, because it is slowly destroying their internal organs and not the muscles. When they get older, they can have kidney and liver problems. Some even die.
Waxes
Waxes are similar to fats except that waxes are composed of only one long-chain fatty acid bonded to a long-chain alcohol group attached. Because of their long, nonpolar carbon chains, waxes are extremely hydrophobic (meaning they lack an affinity for water). Both plants and animals use this waterproofing characteristic as part of their composition. Plants most noticeably use waxes for a thin protective covering of stems and leaves to prevent water loss. Similarly, animals employ waxes for protective purposes; for instance, earwax in humans prevents foreign material from entering and possibly injuring the ear canal area.

Membranes and Membrane Lipids
Lipids are important components of biological membranes.Moreover, These lipids have dual characteristics: part of the molecule is hydrophilic, and part of the molecule is hydrophobic. Membrane lipids may be classified as phospholipids, glycolipids, and/or sphingolipids. Proteins are another important component of biological membranes. Integral proteins span the lipid bilayer, while peripheral proteins are more loosely associated with the surface of the membrane.
Fats and Oils
Fats and oils are composed of molecules known as triglycerides, which are esters composed of three fatty acid units linked to glycerol. Moreover, an increase in the percentage of shorter-chain fatty acids and/or unsaturated fatty acids lowers the melting point of a fat or oil. Moreover, The hydrolysis of fats and oils in the presence of a base makes soap and is known as saponification. Double bonds present in unsaturated triglycerides can be hydrogenated to convert oils (liquid) into margarine (solid).
Polyunsaturated fat
This third fatty acid is simply an unsaturated FA, except hydrogen’s are missing in more than one place. That’s why it’s called polyunsaturated fatty acids.
MORE POSTS:




