bromine and potassium iodide
Hi everyone, thank you for choosing us. have you ever thought about this question “bromine and potassium iodide”.
today on the solsarin site we are going to answer this question.
Stay with us.
Thank you for your choice.


bromine and potassium
The thermal properties of alkali halide crystals. I. The heat capacity of potassium chloride, potassium bromide, potassium iodide and sodium iodide between 2.8 and 270˚K
The measurements were made with two adiabatic calorimeter assemblies, one for the temperature range 2.8 to 20°K and the other for the range 12 to 270°K. The working thermometer for the former assembly was a carbon resistor and for the latter a strain-free capsule-type platinum resistance thermometer. The crystals (pieces approximately 0.5 cm in dimension) were sealed in thin copper calorimeter vessels with a small amount of helium gas to promote heat exchange. The accuracy of the measurements is estimated to be ± 2% at the lowest temperatures, rising rapidly to ± 0.2% for T ⋝ 20°K.
Apparent Debye characteristic temperatures (ΘD) have been calculated for all of the experimental results. The variation of ΘD with temperature is in general accord with the predictions of theory. Starting from the lowest temperatures, ΘD for the three potassium salts first decreases to a minimum in the region of T = ΘD/12, then increases to a maximum in the region of T = ΘD/2 and subsequently decreases smoothly.
Exposure and Exposure Monitoring
Potassium iodide exposure routes are typically through ingestion of potassium iodide tablets or as exposure to iodide through drinking water and food.
The main sources of dietary iodide are seafood (200–1000 μg kg−1) and seaweed (0.1–0.2% iodide by weight). As a pharmaceutical, KI is supplied in 130 mg tablets (each containing 100 mg iodine as iodide) for emergency treatment of the blockade of radioactive iodine uptake, which is a common form of radiation poisoning from environmental contamination of iodine-135 fission products. It also comes as saturated solution of potassium iodide (SSKI), which contains 1000 mg of KI per ml of solution.
Following maternal KI exposure, testing such as fetal ultrasound and neonatal thyroid function tests may be indicated. Discussion with an obstetrician or maternal–fetal medicine specialist is recommended when ultrasound identifies the presence of a fetal goiter as additional monitoring and modifications to the delivery may be warranted.
Effects of Neutral Salts on the Activity of Iodine, Bromine, and Chlorine as Swimming Pool Water Disinfectants
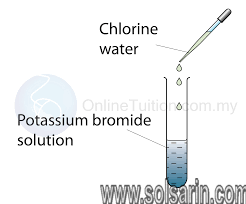

Potassium iodide, potassium bromide, and potassium chloride materially slow down the bacteria killing rates of iodine, bromine, and chlorine, respectively, in distilled water.
The germicidal activity of elemental iodine in water does not increase as the pH is increased from pH 5.8 (the mean value fovind in unbuffered water containing 2.0 ppni free iodine) to 8.0 as might be expected if the germicidal activity of iodine could be attributed to hypoiodous acid. Chlorine is considerably less effective as a disinfectant for sea water than for fresh water when judged by activity against the gram-positive test organism S. faecalis.
While this can be attributed, in part, to interference by the neutral chloride salts present in sea water, the presence of an initial high chlorine demand and difficulties encountered in maintaining available chlorine residuals after this demand has apparently been satisfied make it impossible to conclude that this is the only factor involved.
Reductive cleavages by halide salts
Historically, sodium and potassium iodides were the first halide salts found to dehalogenate halo ketones. The reductions are carried out in acidic media, in order to protonate the ketone.27 The acyclic bromine atom in (15) could be removed in this manner without affecting either the acetoxy group at the same site or the sterically hindered bromine atom at C-12.28 By using metal ions29 or other Lewis acids30 to assist the dehalogenation process, even bromide and chloride salts can be used to effect dehalogenation. Among the best reagents of this type is a combination of SnCl2 and NaCl in aqueous THF.


This substance readily dehalogenates many halo ketones at room temperature.31 Cerium enolates (16) are produced initially; these can be trapped by carbonyl compounds in the medium to form aldols or by D2O to afford the deuterated ketone.
Measurements by the KI-Method
Ambient air is sucked through an aqueous solution which contains potassium iodide. O3 is measured by the oxidation of I− to I2 in aqueous solution which is stabilized by its reaction with thiosulfate S2O32− (Eq. (17)).
The amount of S2O32− consumed is proportional to the O3 present in ambient air. In the standard method, S2O32− is measured by back-titration with I2 using starch as indicator, but also electrochemical detection was employed. Arsenite AsO−3 also can be used instead of thiosulfate which was used in the extended ozone measurements performed from 1876 to 1911 at the Observatoire de Montsouris close to Paris (France). The KI-method was widely used in the 1940s and 1950s.
The KI-method suffers from several interferences, particularly by sulfur dioxide (SO2). Ozone measurements by the KI-method from urban sites must be regarded with some caution because sulfur dioxide is a major air pollutant produced by the combustion of fossil fuels (e.g., of coal). At some stations a CrO3 filter was introduced to remove the bias of SO2, causing possible problems in the homogeneity of the long-term series. Measurements of rural and Alpine sites are much less influenced by such problems. Because of these interferences present monitoring measurements are usually based on the UV-method (see earlier).
Separation, Preconcentration and Spectrophotometry in Inorganic Analysis
Zygmunt Marczenko, Maria Balcerzak, in Analytical Spectroscopy Library, 2000
50.2.3 Starch-iodine method
In a hydrochloric acid medium (optimum concentration ~ 0.5 M HC1), thallium(I) is oxidized with bromine to thallium(III). Thallium(III) oxidizes iodide to iodine, which gives a blue complex with starch. The molar absorptivity of the starch-iodine complex is 3.9·104 (a = 0.19) at 590 nm.
The oxidation of thallium(I) with bromine occurs quantitatively in the cold. The excess of bromine is conveniently removed by reaction with phenol. The formation of a suspension of bromophenol (which interferes in the absorbance measurement) can be avoided, if the excess of bromine is small. The aqueous glycerol solution of starch is stable and gives a good reproducibility of results. The excess of starch does not affect the colour.
OXIDATION OF KETONES, ALDEHYDES, AND CARBOXYLIC ACIDS
James Grimshaw, in Electrochemical Reactions and Mechanisms in Organic Chemistry, 2000

Oxidation of Aldehydes
Electrochemical oxidation of aldehydes to the carboxylic acid, catalysed by bromide or iodide ions, is an extensively exploited process. It is the preferred route for the oxidation of reducing sugars to the aldonic acid and replaces the older technique where bromine in stoichiometric amount was used as oxidising agent[10]. Reaction is carried out in aqueous solution in an undivided cell at a graphite anode using an electrolyte of sodium bromide containing suspended calcium carbonate. The calcium salt of the oxidation product separates. Monosaccharides[11, 12, 13], their methylated derivatives[14] and reducing disaccharides[11] have been converted to the carboxylic acid. Conversion of glucose to gluconic acid is carried out on a technical scale.




