chromium (iii) nitrate and sodium phosphate
Hello dear readers. In this post on Solsarin we are going to talk about ”chromium (iii) nitrate and sodium phosphate“ . Continue reading to find the answer. Please write your comment, Thanks for your attention.
chromium (III) nitrate and sodium phosphate:
Cr(NO3)3 + Na3PO4 → CrPO4 + NaNO3b.
To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above.
- Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F.
- Ionic charges are not yet supported and will be ignored.
- Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will.
- Compound states [like (s) (aq) or (g)] are not required.
- You can use parenthesis () or brackets [].

PROBLEM: Write a molecular equation for the precipitation reaction that occurs (if any) when the following solutions are mixed. If no reaction occurs, write NO REACTION.
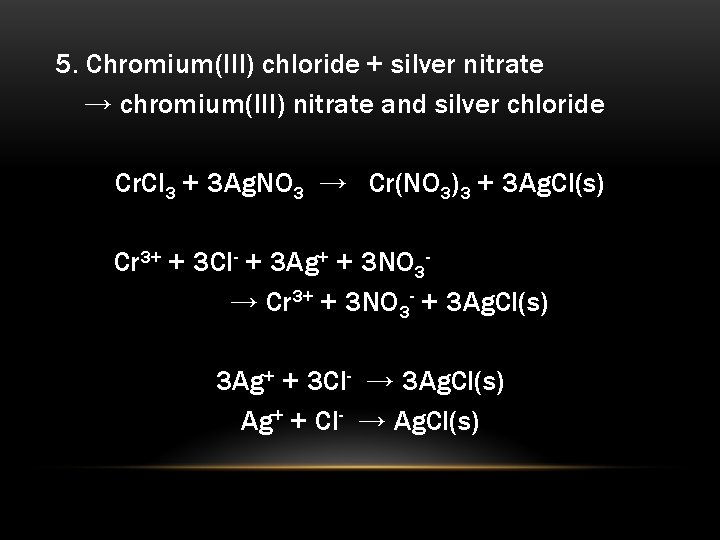
chromium (III) nitrate and sodium phosphatea. Cr(NO3)3 + Na3PO4 → CrPO4 + NaNO3b.
and Cr(NO3)3 + 3Na3PO4 → CrPO4 + 3NaNO3c. Cr(NO3)3 + Na3PO4 → CrPO4 + 3NaNO3d. No reaction.
Answer:
Cr(NO3)3 + Na3PO4 → CrPO4 + 3NaNO3
What scientific concept do you need to know in order to solve this problem?
Our tutors have indicated that to solve this problem you will need to apply the Molecular Equations concept. You can view video lessons to learn Molecular Equations. Or if you need more Molecular Equations practice, you can also practice Molecular Equations practice problems.
What is the difficulty of this problem?
Our tutors rated the difficulty ofWrite a molecular equation for the precipitation reaction th…as medium difficulty.
How long does this problem take to solve?
Our expert Chemistry tutor, Dasha took 3 minutes and 5 seconds to solve this problem. You can follow their steps in the video explanation above.
What professor is this problem relevant for?
Based on our data, we think this problem is relevant for Professor Jones, Auzanneau & deLaat’s class at University of Guelph.
FAQ:
What does chromium III nitrate and sodium phosphate form?
Cr(NO3)2 + Na3PO4 —> Cr3(PO4)2 + NaNO3 —-> Chromium III phosphate and Sodium nitrate
What is chromium III nitrate and sodium phosphate complete ionic equation?
The reaction is:Cr(NO3)3 + Na3PO4 = CrPO4 + 3 NaNO3
What is the balanced equation for chromium III bromide and sodium nitrate?
What is the chemical formula for chromium III phosphate?
Chromium(III) phosphate is CrPO4
Chromium nitrate chemical formula?
Chromium(III) nitrate is Cr(NO3)3
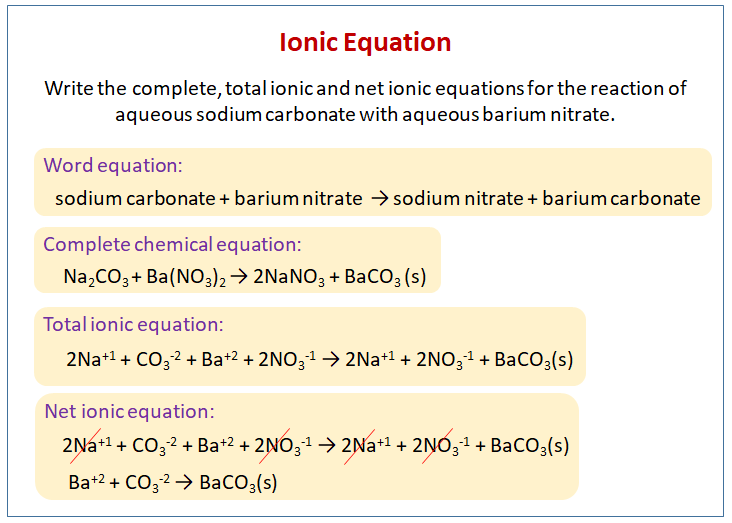

What is the chemical equation for chromium III nitrate and potassium phosphate?
The chemical equation is: Cr(NO3)3 + K3PO4 = CrPO4 + 3 KNO3
What is CrPO4?
chromium(III) phosphate
What is the chemical equation of Chromium III nitrate and potassium phosphate?
The chemical equation is:Cr(NO3)3 + K3PO4 = CrPO4 + 3 KNO3
Iron iii nitrate and sodium iodide?
Produces Iron(III) iodide and Sodium nitrate
What is the formula for chromium III phosphate?
CrPO4
What is the name of CrPO4?
Chromium III phosphate
What is the compound name for CrPO4?
Chromium (III) Phosphate
What is the formula for the compound chromium Nitrate?
Chromium(II) nitrate: Cr(NO3)2Chromium(III) nitrate: Cr(NO3)3
What is the balanced reaction for chromium III bromide and sodium nitrate?
CrBr3 (aq) + 3NaNO3 (aq) —> 3NaBr (aq) + Cr(NO3)3 (s)
React iron iii nitrate and sodium hydroxide?
You get Iron (III) Hydroxide and Sodium Nitrate. It is a double displacement chemical reaction…
What is sodium sulfate and iron III nitrate?
Sodium Sulphate: Na2SO4 Iron (III) Nitrate: Fe(NO3)3
How many atoms are there in one molecule of Cr(NO3)3?
Chromium(III) nitrate is not made of molecules. It is ionic. There are 4 ions in one formula of chromium(III) nitrate.
What is the name of the compound Cr NO3 3?
Chromium (III) Nitrate
What is the correct name for the compound with the formula CrPO4?
chromium(III) phosphate
What is the correct name for the compond with the formula CrPO4?
chromium(III) phosphate
Chemical formula for Chromium III Hydrogen Phosphate?
Cr2(PO4)3
What are the products of iron III nitrate and sodium phosphate?
Fe2NO3 + Na3PO4 —-> NaNO3 + Fe(PO4)
What is the ionic compound name for CrNO33?
Cr(NO3)3 – Chromium III nitrate
Is chromium III Nitrate a solid?
Yes, it is a purple wet looking crystalline solid.
What is the oxidation number of the phosphorus in chromium III phosphate?
Its a secret,you must study harder
What is the chemical formula for the compound formed between chromium(III) and the phosphate ion?
CrPO4
What is the acid base neutralization reaction for chromium III hydroxide plus nitric acid?
Cr(OH)3 + 3HNO3 → Cr(NO3)3 + 3H2O Chromium III hydroxide + Nitric acid → Chromium III nitrate + water
What is the formula for chromium nitrate?
CrNO3 ************2nd Opinion*********** To get the correct formula, you need to state the oxidation number of chromium in the compound, using a Roman numeral. It’s likely to be chromium(III) nitrate, which is Cr(NO3)3
How do you write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate?
2Fe(PO4)+3Na2(SO4)->1Fe3(SO4)2+2Na3(PO4)
Is Chromium 3 Nitrate ionic or covalent?
Chromium (III) nitrate is formed by the reaction between Cr3+ ions and NO3- ions. Thus, forces of attraction due to disparity of charges causes this reaction. As such, we can safely say this is an ionic compound because it is formed by ions.
What is the equation for sodium nitrate and copper III iodide?
the equation for sodium nitrate and copper III iodide can be given below.Cu I 2 +2 Na No3 ->I (NO3)2 + 2NaI. this is the balance reaction for the above.
What happens when iron III nitrate is mixed with sodium chloride?
Nothing. (This is true even if you’re talking about solutions rather than the dry compounds. Both sodium nitrate and iron (III) chloride are soluble, so there’s nothing to drive the reaction.)


Is chromium nitrate ionic or covalent?
Chromium (III) nitrate is formed by the reaction between Cr3+ ions and NO3- ions. Thus, forces of attraction due to disparity of charges causes this reaction. As such, we can safely say this is an ionic compound because it is formed by ions.
What precipitate is formed when Iron III Nitrate and sodium hydroxide react?
Porcupine Balls.
What is the balanced equation of silver nitrate and chromium III chloride?
The balanced equation is: Ag+ (aq) + Cl- (aq) → AgCl(s)
What is the balanced equation for Iron III nitrate plus sodium bicarbonate?
Iron(III) Nitrate + Sodium Bicarbonate —-> Iron(III) Carbonate + Sodium Nitrate + Water + Carbon Dioxide2 Fe(NO3)3 + 6 NaHCO3 —-> Fe2(CO3)3 + 6 NaNO3 + 3 H2O + 3 CO2
What is the balanced equation for Iron III nitrate plus sodium bicarbonate?
Iron(III) Nitrate + Sodium Bicarbonate —-> Iron(III) Carbonate + Sodium Nitrate + Water + Carbon Dioxide2 Fe(NO3)3 + 6 NaHCO3 —-> Fe2(CO3)3 + 6 NaNO3 + 3 H2O + 3 CO2
Is chromium (III) nitrate soluble in water?
Yes it is… according to data supplied by Wikipedia.
What is the balanced equation for chromium III sulfate and calcium Phosphate?
Cr2(SO4)3 + Ca3(PO4)2 —-> 2CrPO4 + 3CaSO4
What is the balanced equation for iron nitrate and sodium hydroxide?
You did not mention whether iron(II) nitrate or Iron(III) nitrate in the question. I assume it as iron(III) nitrate and here is the answer. Fe(NO3)3(aq) + 3 NaOH(aq) –> Fe(OH)3(s) + 3 NaNO3(aq)
What is the formula for chromium(III) sulfide?
The formula for chromium(III) sulfide is Cr2S3.
What is the formula for chromium III arsenate?
The chemical formula of chromium(III) arsenate is CrAsO4.
What is the formula for chromium (III) fluoride?
The formula for chromium (III) fluoride is CrF3.
Can you make chromium III sulfate from chromium III oxide?
Yes, boil Chromium (III) oxide with Sulphuric acid and get the dark green Chromium (III) sulphate solid on evaporation. Cr2O3 + 3H2SO4 —> Cr2(SO4)3 + 3H2O
Cr2S3 chromium two or chromium 3?
It is Chromium III (3)
What is formula of rhodium iii nitrate?
Rhodium III nitrate
Is CrS3 chromium III sulfate?
No. CrS3 is chromium VI sulfide. Chromium III sulfate is Cr2(SO4)3
What is the chemical formula of chromium III bromate?
Chromium III bromate is Cr(BrO3)3
Iron III nitrate covalent or ionic?
Iron(III) nitrate or ferric nitrate is an ionic compound.
What is the formula for chromium III bromide hexahydrate?
The formula for chromium III bromide hexahydrate is CrBr3·6H2O
Write the complete electron configuration for the chromium(III) ion?
The complete electron configuration for the chromium(III) ion is 1s22s22p63s23p64s03d3.
What is the formula for chromium iii thiocyanate?
Cr(SCN)3 is the chemical formula for the Chromium (III) Thiocyanate. This is because Chromium (III) has a charge of +3 while Thiocyanate has a charge of -1. To balance the charges, we switch the 2 charges. So we have 1 ion of chromium (III) with 3 ions of thiocyanate.
What is the chemical name for chromium III chloride?
Another chemical name is chromium trichloride or chromic chloride. Chromium(III) chloride is a chemical name as well.
What is the chemical name for chromium III chloride?
Another chemical name is chromium trichloride or chromic chloride. Chromium(III) chloride is a chemical name as well.
Is iron iii nitrate a molecular or ionic compound?
Iron (III) nitrate is ionic.
Is total chromium the same as chromium III?
Chromium has several valence states, only one of which is Chromium III. States 2, 3, and 6 are most common but 1, 4, and 5 are possible. Total chromium means the amount of chromium in all valence states.
What does chromium react to?
Under still milder conditions, chromium metal reacts with the halogens fluorine, F2, chlorine, Cl2, bromine, Br2, and iodine, I2, to form the corresponding trihalideschromium(III) fluoride, CrF3, chromium(III) chloride, CrCl3, chromium(III) bromide, CrBr3, or chromium(III) iodide, CrI3.
What is the solubility of chromium?
Chromium does not occur freely in nature. The main chromium mineral is chromite. Chromium compounds can be found in waters only in trace amounts. Many chromium compounds are relatively water insoluble. Chromium (III) compounds are water insoluble because these are largely bound to floating particles in water. Chromium (III) oxide and chromium (III) hydroxide are the only water soluble compounds.Chromium (VI) oxide is an example of an excellently water soluble chromium compounds
Is chromium soluble?
Chromium does not occur freely in nature. The main chromium mineral is chromite. Chromium compounds can be found in waters only in trace amounts. Many chromium compounds are relatively water insoluble. Chromium (III) compounds are water insoluble because these are largely bound to floating particles in water. Chromium (III) oxide and chromium (III) hydroxide are the only water soluble compounds.Chromium (VI) oxide is an example of an excellently water soluble chromium compounds
Chemical formula for the compound formed between chromium III and borate?
Chromium(III) borate has the chemical formula CrBO3.
What is the balanced chemical equation Chromium III Sulfate and Sodium Hydroxide?
Cr2(SO4)3 + 6NaOH −−−→ 2Cr(OH)3 + 3Na2SO4
How is chromium made?
Chromium (III) oxide is produced from burning Ammonium
What is the chemical formula for scandium (iii) and phosphate ion?
The chemical formula for scandium (III) and phosphate ion is ScPO4.
What is the product formula of chromium III acetate and tin?
There would be no reaction because tin will not replace the chromium in the chromium (III) acetate. Refer to the related link for an activity series for metals.
What is the balanced chemical equation for Iron III Nitrate and Sodium Hydroxide when the Iron III Nitrate has been distilled in water?
Fe(NO3)3 (aq) + 3 Na(OH) (s) —-> Fe(OH)3 (s) + 3 NaNO3 (aq)




