Compound VS. Molecule
Hello friends. Today we want to talk about “Compound VS. Molecule” in solsarin.
Chemical compound
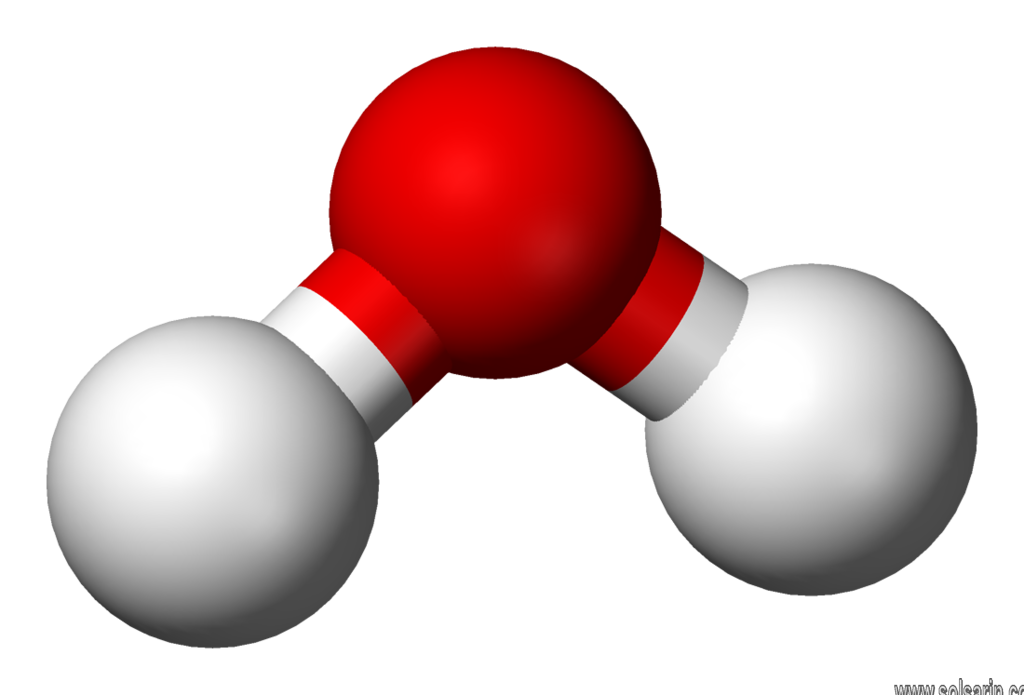

A chemical compound is a chemical substance composing of many identical molecules (or molecular entities) composing of atoms from more than one element holding together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound.
There are four types of compounds, depending on how the constituent atoms hold together:
- molecules held together by covalent bonds
- ionic compounds held together by ionic bonds
- intermetallic compounds held together by metallic bonds
- certain complexes held together by coordinate covalent bonds.
A chemical formula specifies the number of atoms of each element in a compound molecule, using the standard abbreviations for the chemical elements and numerical subscripts. For example, a water molecule has formula H2O indicating two hydrogen atoms bonded to one oxygen atom. Many chemical compounds have a unique CAS number identifier assigned by the Chemical Abstracts Service. Globally, more than 350,000 chemical compounds (including mixtures of chemicals) have been registering for production and use.
A compound can be converting to a different chemical substance by interaction with a second substance via a chemical reaction. In this process, bonds between atoms may be broking in either or both of the interacting substances, and new bonds formed.
Molecule
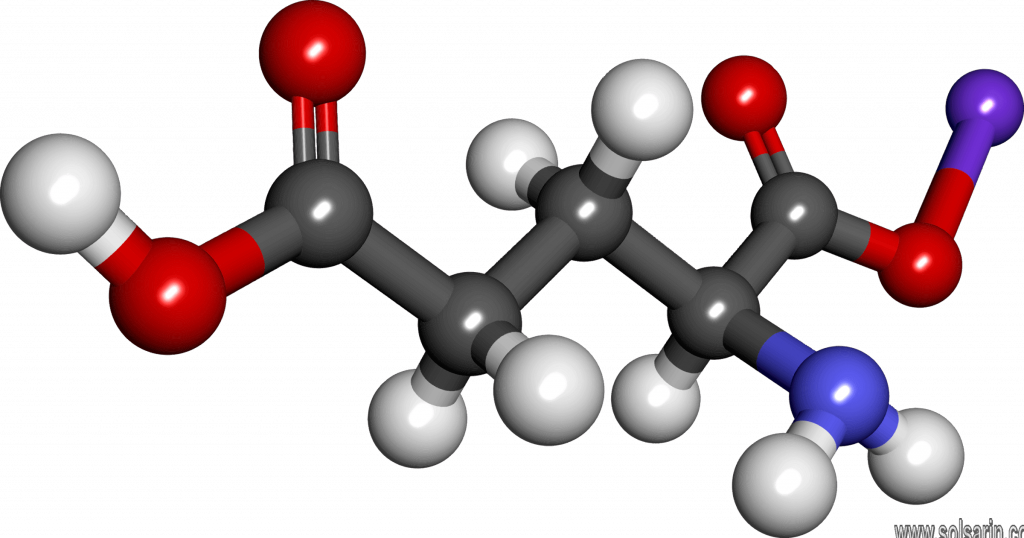

A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.
In quantum physics, organic chemistry, and biochemistry, the distinction from ions is droping and molecule is often using when referring to polyatomic ions.
In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms.
A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O).
Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not considered single molecules.
Molecules as components of matter are common. They also make up most of the oceans and atmosphere. Most organic substances are molecules. The substances of life are molecules, e.g. proteins, the amino acids of which they are composing, the nucleic acids (DNA and RNA), sugars, carbohydrates, fats, and vitamins. The nutrient minerals are generally ionic compounds, thus they are not molecules, e.g. iron sulfate.
However, the majority of familiar solid substances on Earth are made partly or completely of crystals or ionic compounds, which are not made of molecules. These include all of the minerals that make up the substance of the Earth, sand, clay, pebbles, rocks, boulders, bedrock, the molten interior, and the core of the Earth. All of these contain many chemical bonds, but are not made of identifiable molecules.
No typical molecule can be defining for salts nor for covalent crystals, although these are often composed of repeating unit cells that extend either in a plane, e.g. graphene; or three-dimensionally e.g. diamond, quartz, sodium chloride. The theme of repeated unit-cellular-structure also holds for most metals which are condensed phases with metallic bonding. Thus solid metals are not made of molecules.
In glasses, which are solids that exist in a vitreous disordered state, the atoms are held together by chemical bonds with no presence of any definable molecule, nor any of the regularity of repeating unit-cellular-structure that characterizes salts, covalent crystals, and metals.
Difference Between Molecule and Compound
Difference between molecule and compound is one of the most commonly discussing topics in the chemistry subject. In this article, the difference between a molecule and compound in tabular form is giving to help the students understand this topic better. This comparison of molecule and compound can also help the students to know the examples of a compound and molecule and how they are different in their properties.
Before going to the differences, it is important to know the details about a compound and a molecule in detail. By knowing their respective details, it becomes easy to understand their differences. Visit the following links to know about a molecule and compound in detail.
- Introduction To The Concept Of Atoms And Molecules
- Elements And Compounds
Difference Between Molecule and Compound
| Sl. No. | Differentiating Property | Molecule | Compound |
| 1 | Definition | A molecule is a group of two or more atoms held together by chemical bonds. | A compound is a substance which is formed by two or more different types of elements which are united chemically in a fixed proportion. |
| 2 | Relatedness | All molecules are not compounds. | All compounds are molecules. |
| 3 | Example | An example of a molecule is ozone. | An example of a compound is table salt (sodium chloride). |
| 4 | Structure | Molecules are simply a group of atoms which are bonded by a strong force. | All compounds are actual matter in their complete shape. |
| 5 | Visibility | A molecule cannot be seen with the naked eye as they are at the atomic level. | A compound can be easily seen with the naked eye. |
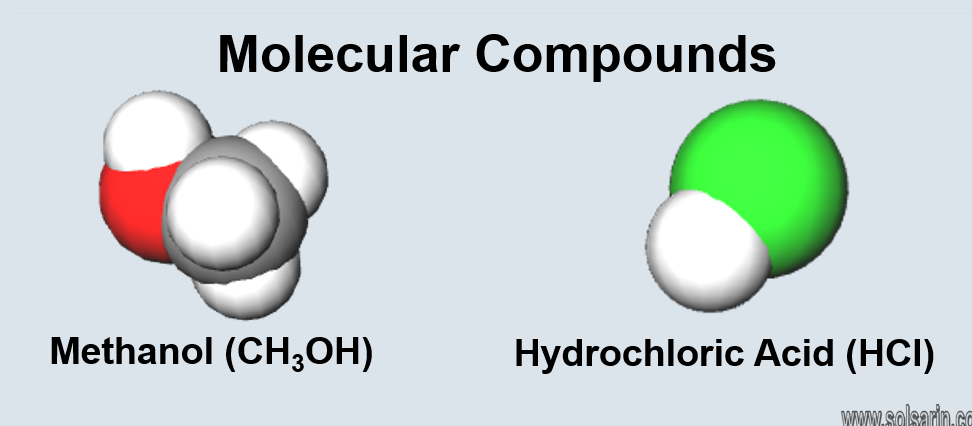

Are all compounds molecules?
Yes, all compounds are molecules. I will elaborate on this answer by giving you an example. Let us consider the molecule of water having a molecular formula of H2O. It is a heteronuclear molecule that has both Hydrogen atoms and an Oxygen atom. But as both these atoms are of different elements, it is also considering as a compound. So if you look at all the molecules, specifically heteronuclear or the ones with atoms of different elements, they also call compounds.
However, not all compounds are molecules. For example, if you look at NaCl or table salt, one cannot consider it as a molecule as Sodium and Chlorine have ionic bonds. And they are also arranging in the ionic lattice, which makes it a compound. Hence all the compounds are molecules, but not all molecules are compounds.
What is an Element
A chemical element is a substance that cannot be breaking down by chemical means. Many different chemical elements have been discovering so far. They have a unique property, i.e., the number of protons in the nucleus. This calls the atomic number. The atomic number of an element is a fixed value for a certain element. Two elements cannot have the same atomic number. Any change in the atomic number changes the element. However, elements can be changing through nuclear reactions.
What is the difference between an element, compound, and molecule?
Elements have only one kind of atom but the others have more than one element chemically bonded.
Explanation:
Elements are one kind of atom. If you have a piece of sodium, every atom is sodium, each having the same number of protons.
A compound has more than one element chemically bonded. This bond is a link between the nuclei of the atoms and the electrons present.
There are three general kinds of chemical bonds : ionic bonds, covalent bonds, and metallic bonds. Depending on which bond is using to make a compound, you might have an ionic compound, a metallic compound (alloy) or a molecular compound (uses covalent bonds).
A covalent bond is when one or more pairs of electrons are SHARING by atoms that are next to each other.
A molecule is a neutral particle (no overall charge) that is holding together by covalent bonds. It is also the smallest part of a molecular compound that keeps the properties (chemical and physical properties) of the compound.
In order to know if something is holding together by covalent bonds, you have to know something about electronegativity but I’m not going to go that far with this answer.
So to sum it up :
element = one kind of atom
a compound is a general term for more than one element chemically bonded
a molecule is a neutral particle holding together with covalent bonds
Compound vs. Mixture
Compounds are pure substances. They are making from the same types of molecules. Each molecule of a compound is making from two or more different kinds of atoms that are chemically bonding. Mixtures make of two or more substances — elements or compounds — that mix physically but not chemically; they do not contain any atomic bonds.

Comparison chart
| Definition | A compound contains atoms of different elements chemically combined together in a fixed ratio. | A mixture is a combination of two or more substances where there is no chemical combination or reaction. |
|---|---|---|
| Composition | Compounds contain different elements in a fixed ratio arranged in a defined manner through chemical bonds. They contain only one type of molecule. Elements that compose the compound are chemically combined. | Mixtures contain different elements and compounds but the ratio is not fixed nor are they combined via chemical bonds. The ingredients are physically mixed but chemically separate. Often they are visibly distinct. |
| Examples | Water (H2O), Sodium chloride (NaCl), Sodium bicarbonate (NaHCO3) and Hydrochloric acid (HCl) are examples of compounds. | Salt in water; pasta and sauce; sand; pebbles; solutions such as rubbing alcohol, dental amalgam, vapor in air; colloids such as mayonnaise, milk, cheese; coarse suspensions such as mud in water, oil in water. |
| Representation | A compound is represented using its chemical formula that represents the symbols of its constituent elements and the number of atoms of each element in one molecule of the compound. | Mixtures cannot be represented by chemical formulas. |
| Chemical and physical properties | Compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose their properties when they combine to make the compound. | Mixtures do not have specific, consistent chemical and physical properties of their own. They reflect the properties of their constituent substances, which retain their original properties. e.g. chocolate milk retains properties of chocolate and milk |
Random Posts