cupric cyanide tetrahydrate
Hello. Welcome to solsarin. This post is about “cupric cyanide tetrahydrate“.
Copper(II) sulfate
Copper(II) sulfate, also known as copper sulphate, are the inorganic compounds with the chemical formula CuSO4(H2O)x, where x can range from 0 to 5. The pentahydrate (x = 5) is the most common form. Older names for this compound include blue vitriol, bluestone,[9] vitriol of copper,[10] and Roman vitriol.[11]
The pentahydrate (CuSO4·5H2O), the most commonly encountered salt, is bright blue. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains.[12] Anhydrous copper sulfate is a light grey powder.
Writing Formulas from Chemical Names:
Ionic compounds have systemic names according to the anion and cation species present in the compound. Oftentimes we are given chemical formulas and asked to reason the names of the compounds but sometimes we have to figure out the chemical formulas of the compounds from the names of the compounds.

Write the chemical formula for cupric(II) cyanide tetrahydrate.
Cupric (II) refers to the Cu2+Cu2+ with valence of magnitude 2.
Cyanide ion is CN−1CN−1 with valence of magnitude 1.
By switching the magnitudes of the valencies and making them subscripts, the formula thus far would be Cu(CN)2Cu(CN)2 i.e. copper takes subscript 1 and cyanide takes subscript 2.
Tetrahydrate means we have to put the water molecules 4H2O4H2O.
The overal formula is Cu(CN)2⋅4H2OCu(CN)2⋅4H2O.
About Copper(II) Formate Tetrahydrate
Copper(II) Formate Tetrahydrate Synonyms

What is the name of cn2?
Cyanogen is the chemical compound with the formula(CN)2. It is a colorless, toxic gas with a pungent odor.The molecule is a pseudohalogen.
what is cyanogen used for?
compounds of nitrogen Cyanogen, or oxalonitrile, (CN)2, isalso used as a chemical intermediate and afumigant.
what is the formula for barium cyanide?
Barium cyanide is a chemical compound with theformula Ba(CN)2.
Is CA CN 2 soluble?
The salts of sodium, potassium and calciumcyanide are quite toxic, as they are highly soluble inwater, and thus readily dissolve to form free cyanide.Operations typically receive cyanide as solid or dissolved NaCN orCa(CN)2.
Abstract
Composition
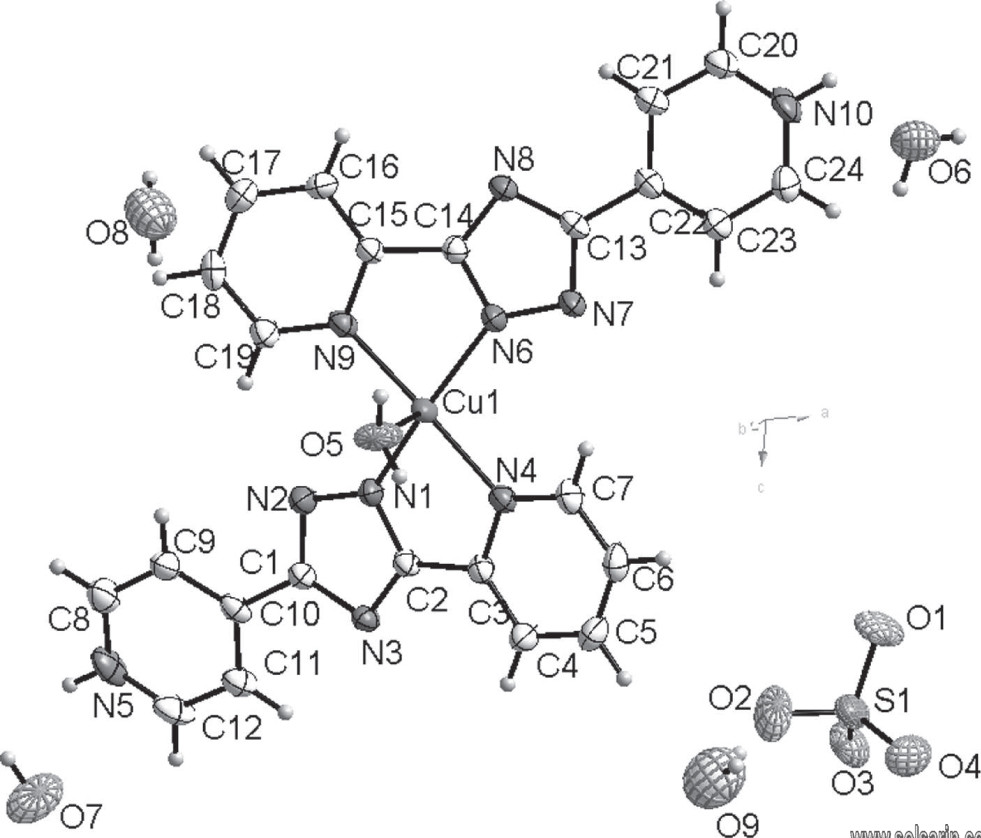
C24H28CuN10O9S, monoclinic, P21/c (no. 14), a = 16.3499(16) Å, b = 14.0411(13) Å, c = 12.7069(13) Å, β = 104.019(2)°, V = 2830.2(5) Å3, Z = 4, Rgt(F) = 0.0472, wRref(F2) = 0.1165, T = 293(2) K.
Source of material
The ligand, 3-(2-pyridyl)-5-(4-pyridyl)-1H-1,2,4-triazole (Hbpt24), was purchased from Alfa Aesar company. Copper sulfate pentahydrate (0.0125 g, 0.05 mmol), Hbpt24 (0.0223 g, 0.1 mmol) and water (20 mL) were placed in 20.0 mL Teflon-lined stainless steel autoclave. It was heated at 383 K for 2 days. After cooling to room temperature, large blue, rod crystals of the title complex were obtained.
Experimental details
The nitrogen and oxygen bound hydrogen atoms were located from the difference Fourier syntheses and refined with restrained distances usind the DFIX comand (O—H: 0.85 Å; N—H: 0.86 Å), and were included with 1.2 Uiso(C, N). The carbon bonded hydrogen atoms were placed on calculated positions using a riding model (AFIX 43 or AFIX 137 option of the SHELX program [2, 3] . A translational pseudosymmetry was detected (b′ = b/2) with a fit of 92% [4].
Discussion
Metal organic complexes are known for their wealth of intriguing architectures, topologies and their potential applications in gas storage, molecular recognition, magnetism, electric conductivity, heterogeneous catalysis, fluorescence probe and much more. My group has contributed to this large field of research some findings in the last years [5], [6], [7], [8].
During our previous work, a number of coordination compounds of 3-(2-pyridyl)-5-(4-pyridyl)-1H-1,2,4-triazole (bpt24) ligand with cobalt and nickel were prepared and structurally characterized [9, 10] . The Ni/bpt24 complexes showed polytropic structures, and a series of Co/bpt24 complexes present crystal transformations from each other.
The asymmetric structure unit of the title compound contains one Cu(II) ion, two bptH ligands, one coordinated water molecule and a free sulfate radical as well as four free water molecules. Of particular note is that the hydrogen atom had shifted from triazolyl nitrogen to pyridyl nitrogen within the bptH ligand. The central atom Cu(II) is chelated by four N atoms from two bptH ligands and is coordinated by one water O atom (Cu—O5, 2.264(3) Å).

Cu—N
The Cu—N bond lengths (1.960(3)−2.264(3) Å) are within the normal range of those in complexes of Cu/N-heterocyclic ligands [11, 12] . The geometry around the Cu(II) atom is best described as a seriously distorted tetragonal pyramid. In addition, the hydrogen bonds formed between the coordinated water molecule and the triazole N atom (O5—H5C⋯N3, 2.820 Å; O5—H5D⋯N8, 2.849 Å), link the title complexes into a one dimensional step-like chain.
The sulfate ions and water molecules form the following hydrogen bonds: O6—H6C⋯O4 (2.868 Å), O6—H6D—O8 (2.714 Å), O7—H7C—O4 (2.910 Å), O7—H7D—O6 (2.848 Å), O8—H8C—O1 (2.940 Å), O8—H8D—O9 (2.690 Å) and O9—H9C—O2 (2.781 Å), O9—H9D—O3 (2.898 Å). The intermolecular hydrogen bonds from pridyl N—H N5—H5⋯O1 (2.608 Å) and N10—H10⋯O3 (2.663 Å), together with the aforementioned ones link all moieties to a supramolecular three-dimensional framework.
Thank you for staying with this post “cupric cyanide tetrahydrate” until the end.