does copper have a giant structure
Hello. Welcome to solsarin. This post is about “does copper have a giant structure”.

Which substance has a giant covalent structure?
Silicon dioxide (often called silica) is the main compound found in sand. It is an example of a substance with a giant covalent structure . It contains many silicon and oxygen atoms. All the atoms in its structure are linked to each other by strong covalent bonds.
Do metals have giant or simple structures?
Metals consist of a giant metallic structure. The atoms in a metal are arranged in a regular pattern and are closely packed together. Metallic bonding – the outer shell electrons become delocalised and surround the positive metal ions. There is a strong electrostatic force of attraction between them.
How do you identify giant structures?
Giant covalent structures
high melting and boiling points. Diamond’s many covalent bonds are strong and substantial energy is needed to break them.
does not conduct electricity. Diamond has no free ions or delocalised electrons to move and carry the charge.
hardness.
Is fullerene a giant covalent structure?
7. Fullerene – form of the elements carbon that can exist as large cage- like structures, based on hexagonal rings of carbon atoms. 8. Giant covalent structure – a huge 3D network of covalently bonded atoms.
Does graphite have a giant structure?
Graphite is another giant covalent structure; that is, a single molecule extending into macroscopic space, but the arrangement of carbon atoms is entirely different in graphite than in diamond. Graphite has a layered structure.
Does silicon have a giant molecular structure?
Silicon – a giant covalent structure
Silicon is a non-metal, and has a giant covalent structure exactly the same as carbon in diamond – hence the high melting point. You have to break strong covalent bonds in order to melt it.
graphene a giant covalent structure?
Giant covalent substances have many atoms joined together by covalent bonds. Diamond, graphite and graphene are forms of carbon with different giant covalent structures.
What are the three giant covalent structures?
giant covalent structures. This page describes the structures of giant covalent substances like diamond, graphite and silicon dioxide (silicon(IV) oxide), and relates those structures to the physical properties of the substances.
Is sodium a giant structure?
The first three are metallic, silicon is giant covalent, and the rest are simple molecules. Sodium, magnesium and aluminium all have metallic structures.
Do metals have a crystal structure?
Materials are made up of a wide variety of atomic structures. However, metals in particular almost always have their atoms organized in a crystalline lattice structure. This means that the atoms of metals are arranged in a patterned, three-dimensional way that repeats itself throughout large portions of the metal.
Why do metals form giant structures?
Metals form giant structures in which electrons in the outer shells of the metal atoms are free to move. The metallic bond is the force of attraction between these free-moving (delocalised) electrons and positive metal ions .
How do you know if a substance has a giant ionic structure?
In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions between the oppositely charged ions. The structure and bonding of ionic compounds explain their properties .
Is a polymer a giant covalent structure?
Giant covalent structures
Polymers are very large molecules made when many smaller molecules join together, end to end. The smaller molecules are called monomers. Polymers have higher melting and boiling points than simple molecules.
Is buckminsterfullerene a giant structure?
Buckyballs are spheres or squashed spheres of carbon atoms. They are made up of large molecules so are not classed as giant covalent networks . Weak intermolecular forces exist between buckyballs.
Is c60 giant covalent?
Buckminsterfullerene. Molecules of C 60 are spherical.


Is graphene a fullerene?
Graphene is an allotrope of carbon that occurs as sheets of carbon while fullerene is an allotrope of carbon which occurs as spheres of carbon. The key difference between graphene and fullerene is that graphene has a two-dimensional structure, while fullerene has a three-dimensional structure.
What is structure of co2?
The carbon dioxide molecule has two double bonds between the carbon and oxygen atoms.
What type of crystal structure does ice have?
hexagonal rings
At standard atmospheric pressure and at temperatures near 0 °C, the ice crystal commonly takes the form of sheets or planes of oxygen atoms joined in a series of open hexagonal rings. The axis parallel to the hexagonal rings is termed the c-axis and coincides with the optical axis of the crystal structure.
What type of structure does diamond have?
giant covalent structure
Does sulfur have a giant structure?
Phosphorus, sulphur, chlorine and argon are simple molecular substances with only van der Waals attractions between the molecules. Their melting or boiling points will be lower than those of the first four members of the period which have giant structures.
Is sulphur a giant covalent?
Silicon Giant covalent lattice Break strong covalent bonds. Phosphorus (P4) Simple molecular (lattice/covalent) Break weak London forces between the molecules. Sulfur (S8) Simple molecular (lattice/covalent) Break weak London forces between the molecules.
Is graphene stronger than diamond?
“Graphene is stronger and stiffer than diamond, yet can be stretched by a quarter of its length, like rubber,” said Andre Geim, who shared the 2010 Nobel prize in physics with Kostya Novoselov for their discovery of graphene.
Which among the following is are examples of giant molecule?
, diamonds, and graphite are four examples of giant molecules. Diamond and graphite come under the category of element carbon forms. These two are allotropes of Carbon because they exist in the same physical state.
How do you know if a compound is giant or simple?
If it is a gas, liquid or low melting point solid then you are talking about a simple molecular substance. Full stop! If it is a high melting point solid, it will be a giant structure – either ionic, metallic or giant covalent.


Does Iron have a giant structure?
The structure of iron is an example of a giant molecule. The atoms of iron are held together by ionic bonds. Iron conducts electricity because iron atoms move through the solid.
Is methane simple molecular structure?
Hydrogen, ammonia, methane and pure water are also simple molecules. All have strong covalent bonds between their atoms, but much weaker intermolecular forces between molecules.
Is carbon a giant molecular structure?
The surrounding carbon atoms can still form more single covalent bonds with yet more carbon atoms. This will extend the network of covalent bonds between many carbon atoms, hence establishing a giant molecular structure.
What is copper crystal structure?
The metal, copper, has a face centered cubic crystal structure.
Why Pure copper is malleable?
When a metal has these kinds of electrons—for example iron, aluminium, and copper—they’re highly malleable when heated, because the atoms are able to easily slide over each other2, allowing us to hammer them into useful shapes.
What makes copper ductile?
Metals are described as malleable (can be beaten into sheets) and ductile (can be pulled out into wires). This is because of the ability of the atoms to roll over each other into new positions without breaking the metallic bond.
Why is copper a good conductor of electricity GCSE?
they are electrical conductors because their delocalised electrons carry electrical charge through the metal. they are good conductors of thermal energy because their delocalised electrons transfer energy.
Do ionic compounds form giant structures?
An ionic compound is a giant structure of ions. The ions have a regular, repeating arrangement called an ionic lattice .
Which substance has a giant covalent structure?
It is an example of a substance with a giant covalent structure . It contains many silicon and oxygen atoms.
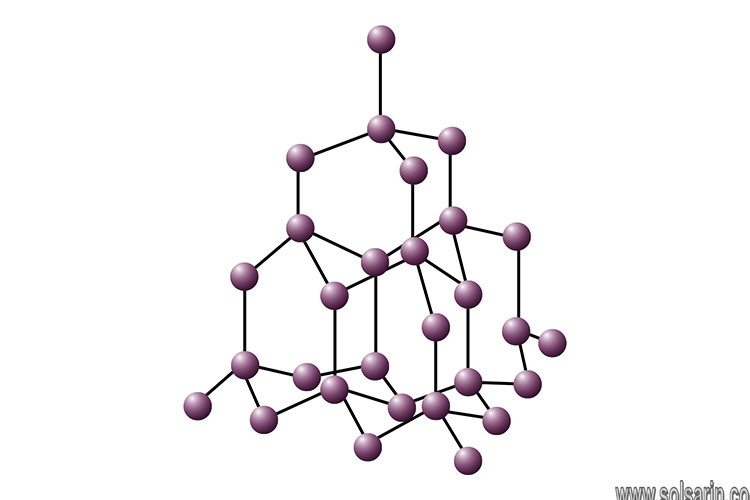

What makes a giant structure?
Giant covalent structures contain very many atoms , each joined to adjacent atoms by covalent bonds .



