does methanol conduct electricity?
Hi, welcome to solsarin site, in this post we want to talk about“does methanol conduct electricity”,
thank you for choosing us.
does methanol conduct electricity?
In reality, a solution of methanol and water does conduct electricity, just to a MUCH lower extent than a solution of HCl in water. The self-ionisation constant of methanol will be very low, it will be only marginally different to that of water.
Methanol, formula CH3OH, is a colorless, fairly volatile liquid with a faintly sweet pungent odor, similar, but somewhat milder and sweeter than ethanol.
Methanol is toxic, and may cause blindness. The vapors are slightly heavier than air and may explode if ignited.
Methanol is used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products.
Does pure methanol conduct electricity?
So for methanol and ethanol the self-dissociation into ions will be weaker than in water. These ions will still be present, though, and will conduct electricity even in pure ethanol and methanol solutions, but the conductivities will be very low.


Non-electrolytes:
The study of chemistry involves the study of compounds that can be considered electrolytes or non-electrolytes. Electrolytes are usually compounds that dissociate into their ion components (usually cations and anions) in an aqueous solution that is capable to conduct electricity. On the other hand, non-electrolytes are compounds that are not capable to dissociate into ions in a solution and cannot conduct electricity.
What two things Cannot conduct electricity?
Nonmetals are elements that generally cannot conduct electricity. Examples of nonmetals include hydrogen, carbon, chlorine, and helium. Properties of nonmetals include a relatively low boiling point, and poor conductivity; solid nonmetals are dull and brittle.
What is methanol conductivity?
methanol.org 6. By comparison, the electrical conductivity of neat methanol is 44 x106 pS/m. 19. (44 µS/m) and that of industrial grade. methanol is 30 µS/m.
Is methanol a weak electrolyte?
Methanol, CH3OH, is a non-electrolyte; oxalic acid, H2C2O4, is a weak electrolyte; and sodium chloride, NaCl, is a strong electrolyte.
Does methanol dissolve in water?
alcohols. …is referred to as a hydrophilic (“water-loving”) group, because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. Methanol, ethanol, n-propyl alcohol, isopropyl alcohol, and t-butyl alcohol are all miscible with water.
What happens if electricity touches water?
Water is an excellent conductor of electricity. You can become electricity’s path to the ground if you are touching water that touches electricity. Electricity would travel through the water and through you to the ground.


What happens when a weak electrolyte dissolves in water?
Weak electrolytes only partially ionize in water (usually 1% to 10%), while strong electrolytes completely ionize (100%). Consequently, what happens when a weak electrolyte dissolves in water? Water is a solvent. If it is a strong solute, the water will surround all the different ions making it fully ionized.
Is Methanol Soluble In Water?
How do you know if a solution will conduct electricity?
Metals conduct electricity, so if there is just the symbol of a metal, eg Mg(s), then it will conduct, even in the solid or liquid state. 2. Ionic compounds conduct when they are dissolved in water, or are melted. Ionic compounds always start with a metal.


Uses & Benefits
Antifreeze
Methanol has chemical properties which allow it to lower the freezing point of a water-based liquid and increase its boiling point. These attributes lead methanol to be used as an antifreeze in windshield washer fluid to keep the cleaning fluid from freezing. It is also injected in natural gas pipelines, where it lowers the freezing point of water during oil and gas transport.
Solvent
Methanol is primarily used as an industrial solvent to help create inks, resins, adhesives, and dyes. It is also used as a solvent in the manufacture of important pharmaceutical ingredients and products such as cholesterol, streptomycin, vitamins and hormones.
Fuel
Roughly 45 percent of the world’s methanol is used in energy-related applications. Methanol can be used as a type of vehicle fuel or marine fuel for boats. It can also be blended into gasoline to produce an efficient fuel known as methyl tertiary butyl ether (MTBE) which can have lower emissions than conventional gasoline. Methanol also is used in biodiesel, a renewable type of fuel made from plants or animal fats that can be used in place of, or
blended into, conventional fuel.
Food
Methanol occurs naturally in many foods, including fruits and vegetables. Dietary methanol helps to regulate human gene activity . It is also created in the human digestive system to help metabolize food.
Methanol vs. Ethanol Chemically
Chemists have made their life considerably simpler by establishing a standard vocabulary for describing molecules, ensuring that everyone knows what they are talking about. The name of a molecule describes the number of carbons it contains. Moreover, According to Chemistry School, the prefixes “meth-” and “eth-” in chemistry refer to the number of carbon atoms in the molecule’s root chain. Moreover, The suffix “-ol” appears at the end of the names of chemicals of the alcohol family.Methanol has the chemical formula CH3OH and has one carbon in its root chain., which means it has one carbon atom and four hydrogen atoms, one of which is bonded to an oxygen.
Ethane’s chemical formula is C2H5OH, which means it has two carbon atoms in its root chain and six hydrogen atoms outside of it, one of which is bonded to oxygen.Ethanol issometimes known as ethyl alcohol, however, this is a technical word for what is usually known as “alcohol” in the form of spirits, whiskey, wine, beer, and other beverages. Methyl alcohol is another name for methanol. Methanol is used as a solvent, a raw ingredient in the manufacture of compounds such as formaldehyde, and for cleaning metals, according to Britannica. Drinking methanol is clearly a poor idea.
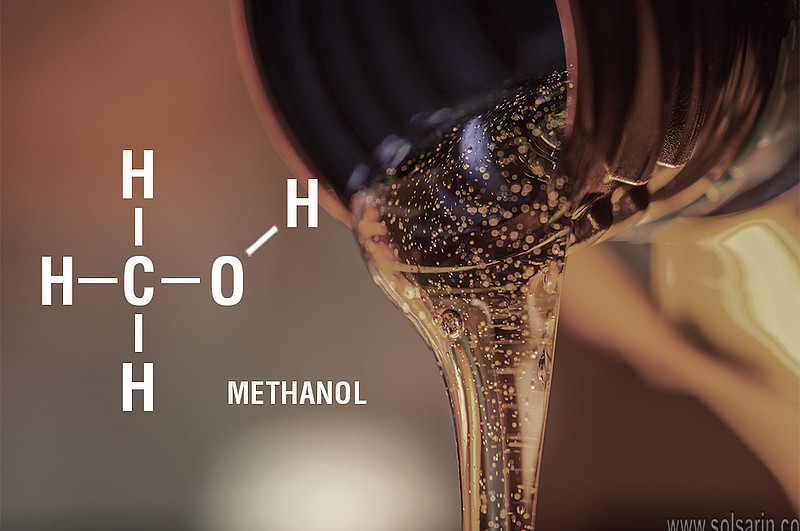
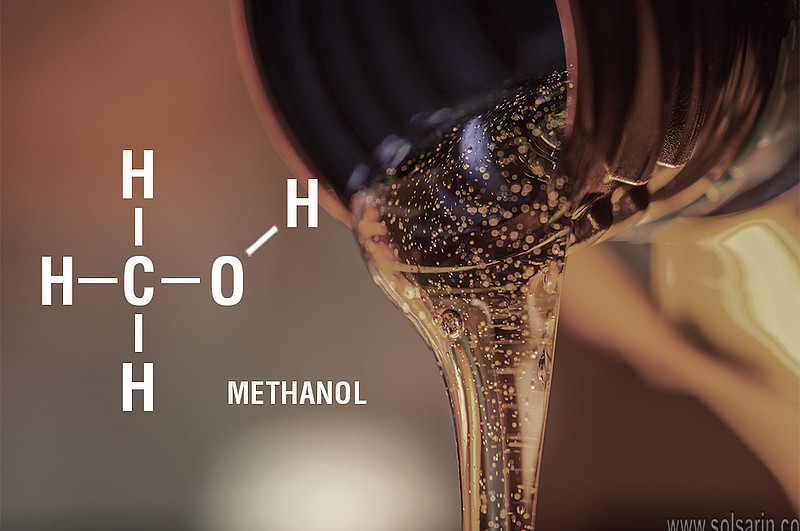
Difference between Ethanol and Methanol |
|
| Ethanol is a type of alcohol with its carbon skeleton consisting of an ethyl ring. | Methanol consists of in its carbon bond methyl group. |
| Ethanol is a poor acid compared with water, in terms of acidity. | methanol is higher acidic than water |
| Ethanol has a heavy, burning smell and emits bright blue flame. | Methanol is unpredictable and has a characteristic odour. When burning it gives off light white flame. |
| Ethanol is typically prepared by the fermentation of food crops from factories. | Methanol is manufactured mainly by synthetic processes. |
| Ethanol is the primary ingredient of alcoholic beverages. | Since methanol is highly poisonous it is not appropriate for use at all. Generally used in the manufacturing of products such as formaldehyde etc. |
does alcohol conduct electricity?
No, alcohol does not conduct electricity because it is a covalent compound. Therefore, it does not have free electrons to flow across it. Being, a stable compound, it does not ionize in water because bonding within the alcohol molecule is strong enough to avoid the breaking of bonds by the water molecules. So, alcohol does not conduct electricity.
Basically, alcohol is ethanol or ethyl alcohol having chemical formulae C2H5OH. Consumption of alcohol is legal in major parts of the world. Consumption of alcohol in lesser quantity reduces anxiety.
Whereas consuming higher doses of alcohol has a bad effect on health. It can cause unconsciousness, drunkenness and even death.
If we talk about the chemical structure of an alcohol molecule.Moreover, It has chemical formulae written as C2H5OH or CH3CH2OH. It contains one Methyl group (CH3) and one Methylene group (CH2) and one Hydroxyl group (OH).
The molecular mass of C2H5OH = 6 mol of hydrogen atoms (6 × 1.0079 g), 2 mol of carbon atoms (2 × 12.011 g), and 1 mol of oxygen atoms (1 × 15.9994 g) = 46.07 g/mol.
MORE POSTS: