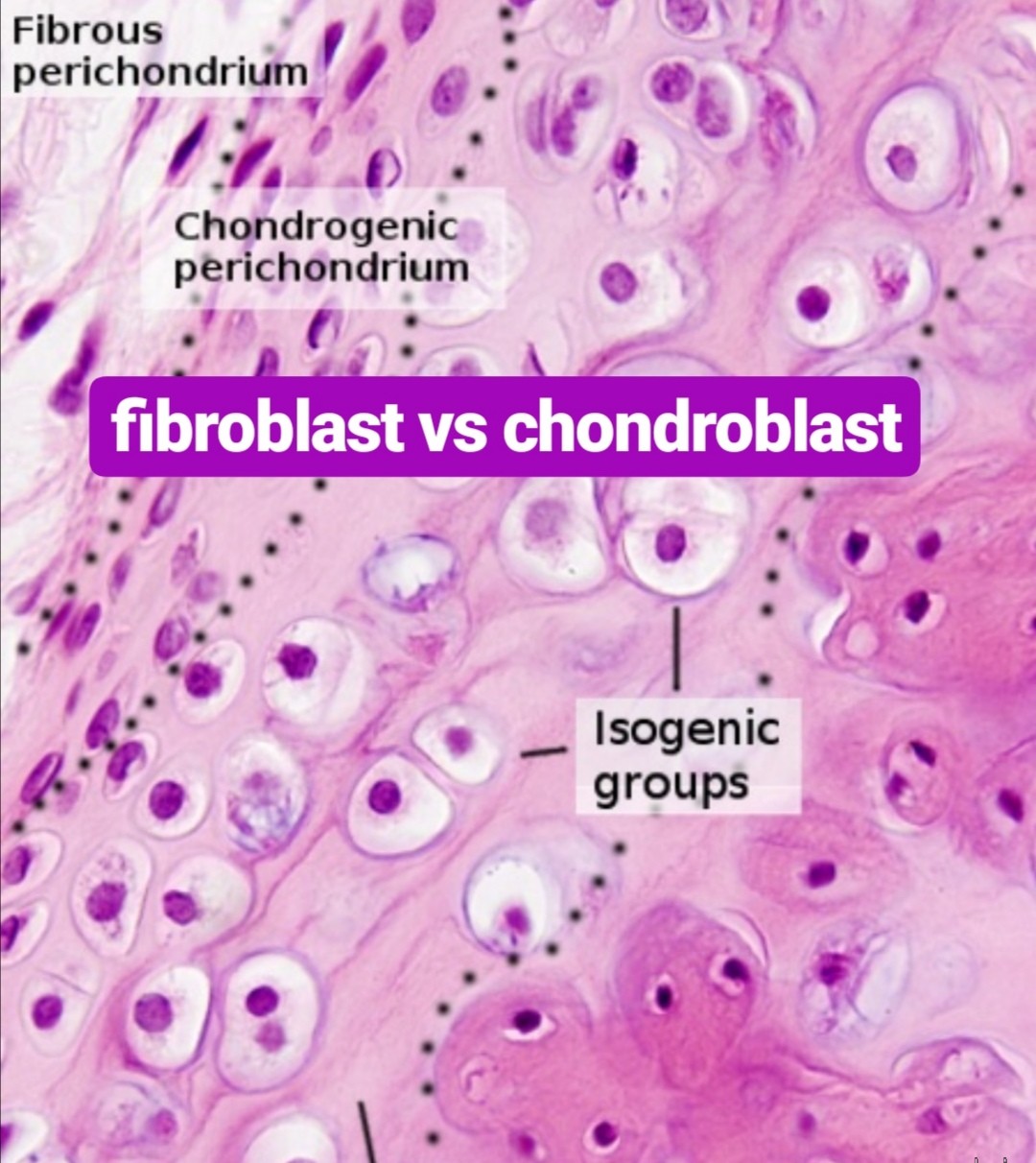
fibroblast vs chondroblast
Hi,welcome to solsarin, today we want to talk about“fibroblast vs chondroblast”,
thank you for choosing us.
fibroblast vs chondroblast
As nouns the difference between fibroblast and chondrocytes is that fibroblast is a cell found in connective tissue that produces fibers, such as collagen while chondroblast is a cell which originates from a mesenchymal stem cell and forms chondrocytes.
fibroblast (Noun)
a cell found in connective tissue that produces fibers, such as collagen.
chondroblast (Noun)
- A cell which originates from a mesenchymal stem cell and forms chondrocytes.

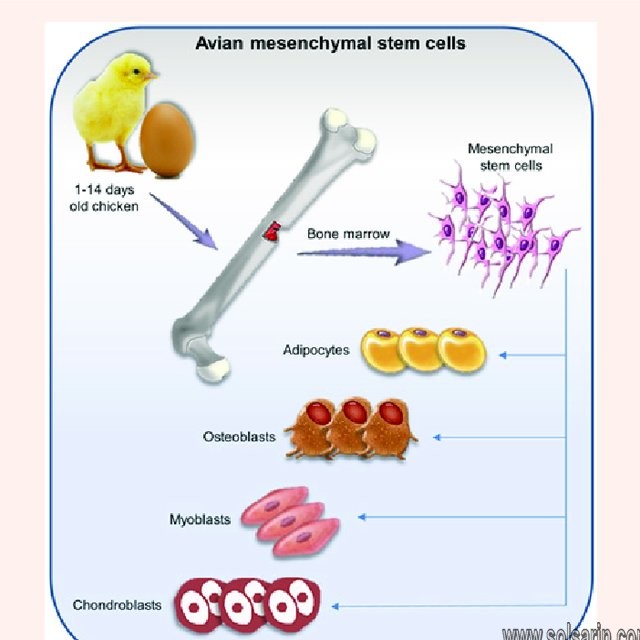
What are chondroblasts?
Chondroblasts are an immature type of cells found in the cartilage. they are also known as perichondrial cells. they are a type of mesenchymal progenitor cells. They secrete the extracellular matrix of the cartilage. The extracellular matrix of the cartilage is composed of collagen, hyaluronic acid, glycoproteins, proteoglycans, and water. Chondroblasts can be found in the perichondrium of the cartilage. Perichondrium is the thin layer of connective tissue, which protects the cartilage. When needed, the chondroblasts are activated by hormones such as growth hormones and thyroid hormones and secrete the extracellular matrix. This increases the size of the cartilage. This type of growth in cartilage is called the appositional growth.
The extracellular matrix secreted by chondroblasts can be found in the outer covering of the cartilage. Once chondroblasts are trapped inside the extracellular matrix, the cells become chondrocytes.
What do chondroblasts do?
Chondroblasts contribute to the formation of the extracellular matrix and are the precursors of the chondrocytes, which collectively make up cartilage. Chondroblasts secrete the extracellular matrix which is composed of various substances, including collagen, proteoglycans, glycoproteins, hyaluronic acid, water, and macromolecules. These substances provide strength and structural support to the developing cartilage. In addition, chondroblasts mature into chondrocytes, which make up the cellular components of cartilage. These cells also contribute to the appositional growth of cartilage, which is characterized by the thickening of existing cartilage. They do this by secreting the extracellular matrix at the peripheral cartilage surfaces.
In existing cartilage, chondrocytes can be damaged or destroyed. When this happens the remaining chondrocytes differentiate into chondroblasts in order to secrete more extracellular matrix and regenerate the lost cartilage tissue. However, this cartilage regeneration process is very slow, in part because of the lack of adequate blood supply.
In some cases, unregulated chondroblast growth and function can lead to the formation of chondroblastomas or chondrosarcomas. Chondroblastomas are benign tumors that form at endochondral ossification sites (places where growing cartilage is replaced by bone). They most commonly occur on the thigh bone (femur), shinbone (tibia), or humerus, located in the upper arm. On the other hand, chondrosarcomas are malignant tumors originating from the chondroblasts, and make up about 30% of bone cancer cases.

What type of cell is a Chondroblast?
Chondroblasts are young, immature cartilage cells that eventually form chondrocytes via a process of chondrogenesis. Chondroblasts are also known as subchondral cortico-spongious progenitors or perichondrial cells.
Is Chondroblast mature or immature?
Chondroblasts are an immature type of cells found in the cartilage. they are also known as perichondrial cells. they are a type of mesenchymal progenitor cells.
Matrix formation and composition
The chondroid matrix (i.e., the ground substance of the cartilage) is made up of collagen, proteoglycans, glycoproteins, hyaluronic acid, water, and macromolecules that are secreted by the chondroblasts. moreover, These components provide characteristic strength, structure, and tensile strength to the cartilage.
ECM is a gelatinous structure that can absorb water like a sponge. ECM posses unique mechano-physical properties, namely:
- Mechanical property, i.e., strength, structure, and tensile strength of the cartilage, is due to the presence of collagen in it
- Physical property, i.e., swelling and elasticity of the cartilage, is due to the presence of the proteoglycans, primarily aggrecan. The presence of such components makes cartilage a highly hydrous structure.
Chondronectin binds glycosaminoglycans & collagen fibers, which, in turn, provides adherence to chondrocyte and chondroblasts.
What are Fibroblasts?
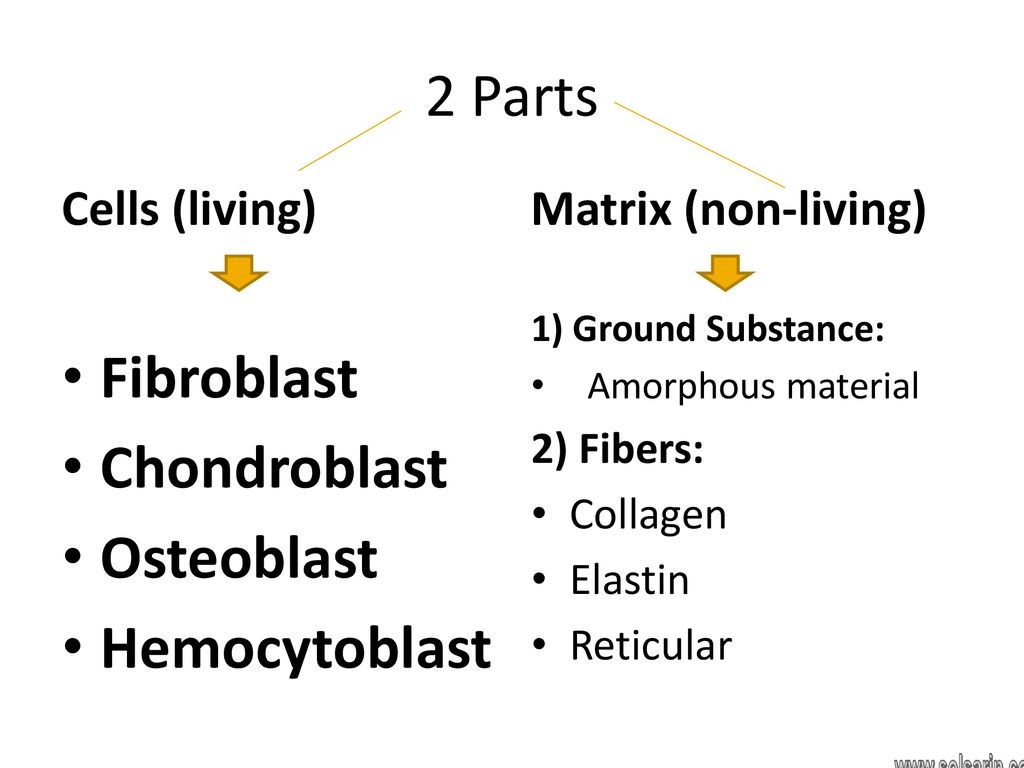
fibroblast, the principal active cell of connective tissue. Fibroblasts are large, flat, elongated (spindle-shaped) cells possessing processes extending out from the ends of the cell body. The cell nucleus is flat and oval. Fibroblasts produce tropocollagen, which is the forerunner of collagen, and ground substance, an amorphous gel-like matrix that fills the spaces between cells and fibres in connective tissue.
Fibroblasts appear to play an important role in wound healing, and this activity is thought to be regulated by cells known as fibrocytes residing in the tissue stroma. Following tissue injury, fibroblasts migrate to the site of damage, where they deposit new collagen and facilitate the healing process.

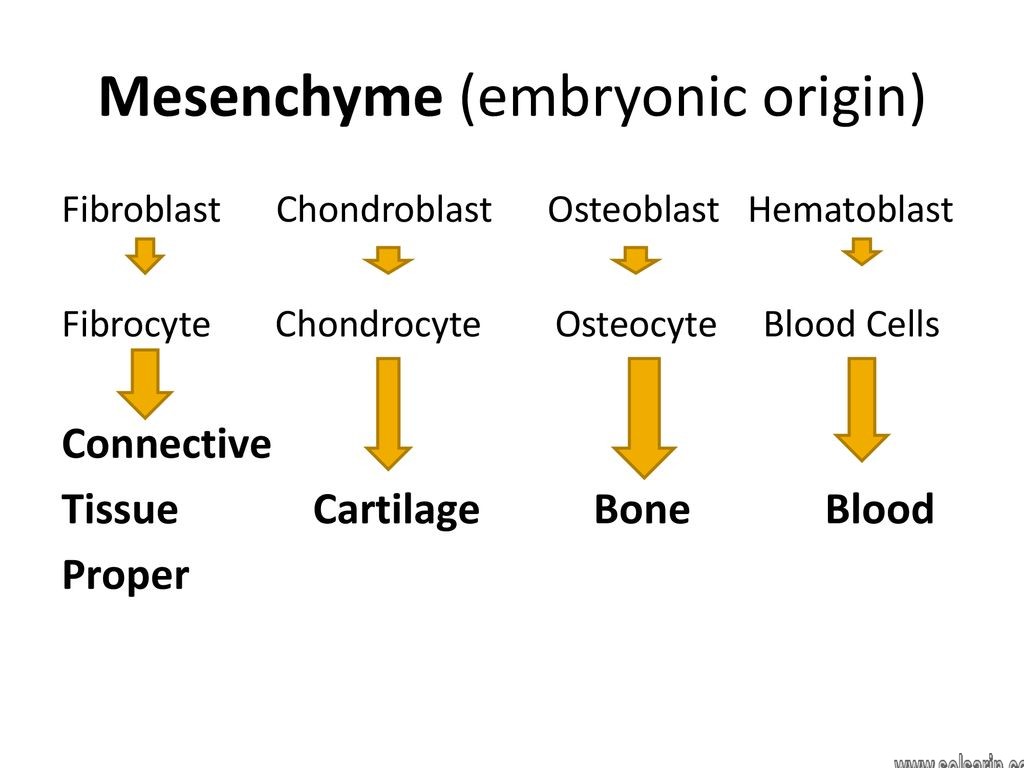
Fibroblast origin
The primary function of fibroblasts is the maintenance of structural integrity within the connective tissue. They achieve this by secreting extracellular matrix precursors required for formation of the connective tissue and various fibres.
Fibroblasts are originally derived from primitive mesenchyme and therefore display the filament protein vimentin, which acts as a marker of mesodermal origin. In some cases, epithelial cells may also produce fibroblasts, a process which is referred to as epithelial–mesenchymal transition (EMT). Conversely, fibroblasts sometimes undergo mesenchymal–epithelial transition (MET) to produce epithelia, a process that is seen in development, tissue repair and tumor growth.
Introduction
Fibroblasts are the workhorse of the most important tissue that holds the human body together—connective tissue. Connective tissue joins and supports all other tissues, including the parenchymal tissues of organs. This connective tissue is made of fibroblasts widely-spaced in a vast extracellular matrix (ECM) of fibrous proteins and gelatinous ground substance. Fibroblasts produce the ECM’s structural proteins (e.g., fibrous collagen and elastin), adhesive proteins (e.g., laminin and fibronectin), and ground substance (e.g., glycosaminoglycans, such as hyaluronan and glycoproteins).
However, fibroblasts also play various additional roles beyond ECM production. For example, fibroblasts serve pivotal roles in ECM maintenance and reabsorption, wound healing, inflammation, angiogenesis, cancer progression, and in physiological as well as pathological tissue fibrosis. Ancillary to these various biological roles, fibroblasts produce and respond to a broad array of paracrine and autocrine signals, such as cytokines and growth factors. Targeting these ancillary signaling events is the main strategy underlying multiple lines of research for a new generation of treatments for fibroblast-related disorders.
Introduction
Fibroblasts are mesenchymal cells derived from the embryonic mesoderm tissue, and they are not terminally differentiated. They can be activated by a variety of chemical signals that promote proliferation and cellular differentiation to form myofibroblasts with an up-regulated rate of matrix production.
Fibroblast activation plays a vital role in wound healing however, in some cases and for reasons that remain to be fully elucidated, their activation becomes uncontrolled, producing a pathological fibrotic response that promotes multiple diseases and affects a variety of organs. Indeed, fibrosis plays a significant contributory role in most cases of organ failure. Examples are wide ranging: systemic sclerosis (SSc); idiopathic pulmonary fibrosis (IPF); liver cirrhosis; kidney fibrosis; and the cardiac fibrosis observed in cardiac hypertrophy resulting in heart failure. The essential role of fibroblasts in lung fibrosis was validated using lineage-specific deletion of the type II TGFβ receptor (Hoyles et al., 2011).
Here we review a variety of fibroblast functions that illustrate a central role for fibroblasts in the pathology of fibrosis.moreover, We review ECM production in relation to fibrosis, some examples of critical chemical signaling, myofibroblast differentiation, the role of fibroblasts in stromal-cancer interactions, and potential clinical therapies targeting fibroblasts.

Cancer-Associated Fibroblasts
Cancer-associated fibroblasts (CAFs) are a subgroup of activated fibroblasts that are found within the tumor microenvironment (TME). moreover, CAFs contribute to cancer progression, metastasis, and immune cell reprogramming by secretion of growth factors, cytokines and chemokines, interleukins, matrix metalloproteases (MMPs), and ECM deposition.
Several mechanisms have been shown to activate normal, resident fibroblasts to become CAFs including:
- Contact with cancer cells
- Changes or remodeling in the ECM causing stromal stiffness
- DNA damaging chemotherapy, radiotherapy, or targeted therapy
- Physiological stress and reactive oxygen species (ROS) production
- Growth factor signaling and receptor tyrosine kinase ligand expression such as TGF-β, HGF, PDGF, and FGF
- Exposure to inflammatory signals including IL-1β, IL-6, and TNFα
Proteins and Ground Substance
Beneath a microscope, fibroblasts are fairly nondescript. Fibroblasts that are actively producing connective tissue are a bit larger than inactive, or ‘resting,’ fibroblasts, but even active fibroblasts are visually uninteresting. However, few cells have functions that are as fascinating as fibroblasts, nor are many cells as adaptable to different situations.
Depending on where they are and what type of connective tissue they’re building, fibroblasts can secrete several different types of fibrous proteins:
Collagen is a tough, strong fiber that provides tensile and compressive strength to your organs and tissues. Collagen is the most abundant protein in the human body. If you removed the cells from one of your organs, a collagen ‘skeleton’ of that organ would be left behind. Collagen is the major constituent of your tendons and ligaments, and much like the tough backing on a carpet, it provides support for your skin. When you have an injury to your skin, collagen is the stuff that forms the scar. Due to the many tasks required of connective tissue, your fibroblasts ‘know’ how to make at least a dozen different types of collagen.
Elastin is a stretchy and resilient protein. Like a rubber band, elastin helps tissues return to their previous shape after they’ve been stretched. In sun-damaged skin, elastin fibers become weak and disorganized, allowing the skin to droop and sag.