formula weight vs molecular weight
Hi, welcome to solsarin site, in this post we want to talk about“formula weight vs molecular weight”,
thank you for choosing us.
formula weight vs molecular weight
Atoms join and make molecules. Atoms can join in various combinations to form molecules, and for our study purposes, we have certain ways of indicating the molecules. Molecular formulas are different types. Before talking about formula weight or molecular weight, it is necessary to know what
are a molecular formula and an empirical formula.
Molecular formula is the formula which shows all the atoms in a molecule. For example, the molecular formula of glucose is C6H12O6. So a glucose molecule contains six carbon and oxygen atoms and twelve hydrogen atoms. Empirical formula shows the simplest ratio of the number of atoms in a molecule. For example, CH2O is the empirical formula of glucose. For some molecules like water (H2O), empirical formula and the molecular formula are the same.


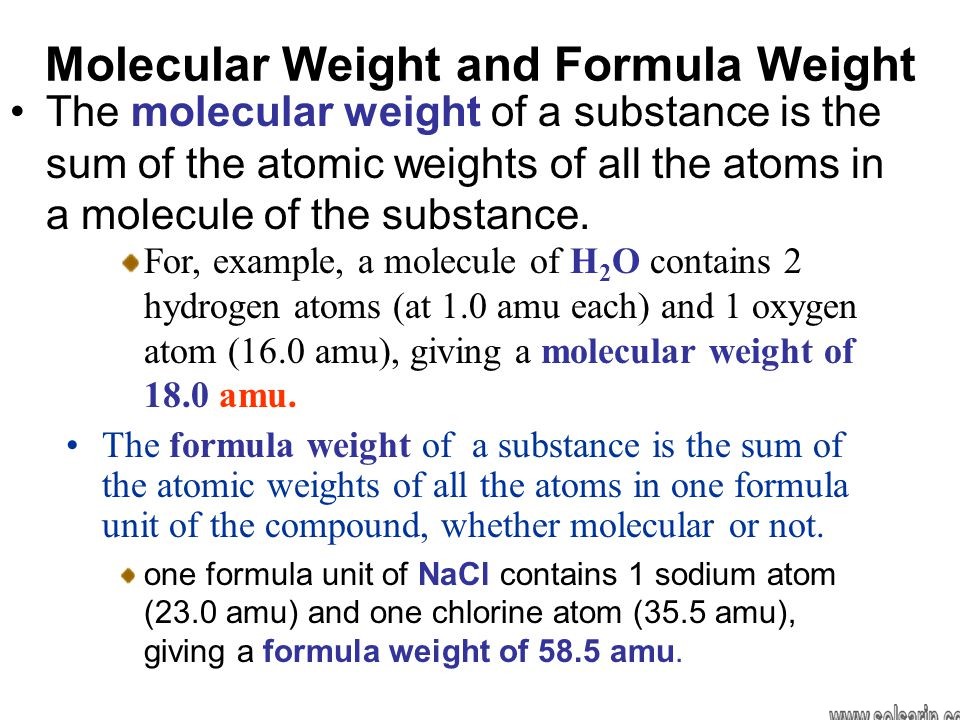
Formula and Molecular Weights
The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. For example, water (H2O) has a formula weight of:
If a substance exists as discrete molecules (as with atoms that are chemically bonded together) then
the chemical formula is the molecular formula, and the formula weight is the molecular weight. For example, carbon, hydrogen and oxygen can chemically bond to form a molecule of the sugar glucose
with the chemical and molecular formula of C6H12O6. The formula weight and the molecular weight of glucose is thus:
Ionic substances are not chemically bonded and do not exist as discrete molecules. However, they do associate in discrete ratios of ions. Thus, we can describe their formula weights, but not their molecular weights. Table salt (NaCl
), for example, has a formula weight of:
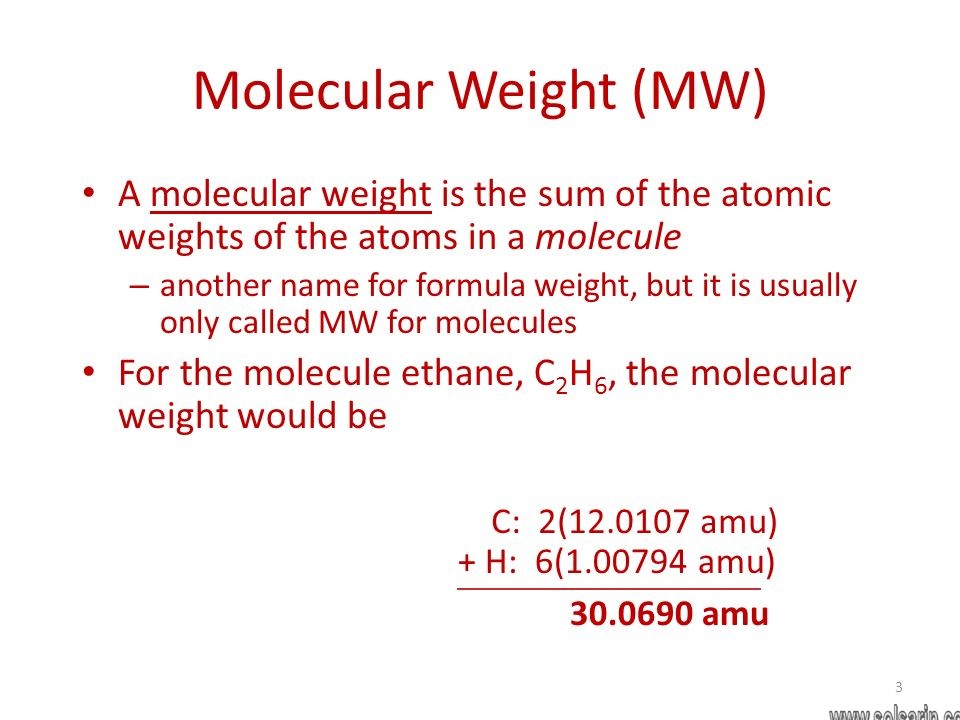
Molecular Weight
The term molecular weight defined as the mass of a molecule. It is also known as the relative molecular mass is another because molecular weight calculated as the mass that is relative to the carbon -12 isotopes.
The smallest particle in an element or compound is a molecule that possesses the chemical properties of that element or compound. They are made up of atoms join together by chemical bonds.
Molecular Weight of a substance = MassofonemoleculesofsubstancesMassofonecarbon−12atom x 112
The molecular weight has no units because the division has done between two masses that have the same units. Therefore, the unit of the molecular weight taken as atomic mass units or amu.
With the help of the above equation, we can find the molecular weight of an element or a compound. In other words, we can say that the Molecular weight of a particular molecule is equal to the sum of the atomic masses of each element.

How Do You Calculate Molecular Weight?
First, you will need the mass of individual compounds. This number is found under the element on the period table. For example, the molecular weight of oxygen is 16.00 amu.
Once you know the mass of the individual components of a compound, you can find the molecular weight.
For example, the molecular weight of water (H2O) is:
The mass of hydrogen is 1.008 amu, and the mass of oxygen is 16.00 amu. Plugging that in yields:
Adding the molecular weights of each element together gives:
Thus, the molecular weight of water is 18.02 amu.
formula weight
formula weight, in chemistry, a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a formula by the number of atoms of that element present in the formula, and then adding all of these products together. For example,
the formula weight of water (H2O) is two times the atomic weight of hydrogen plus one times the atomic weight of oxygen. Numerically, this is (2×1.00797)+(1×15.9994)=2.01594+15.9994=18.01534. If the formula used in
computing the formula weight is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
For example, the weight percentage of hydrogen in water is determined by taking two times the
atomic weight of hydrogen, dividing it by the formula weight of water, and multiplying by 100. Numerically, this is 100×(2×1.00797)/18.01534=11.19% hydrogen in water by weight. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
formula weight
For example, it is known that two molecules of hydrogen gas, H2, react with one molecule of oxygen gas, O2, to form two molecules of water, H2O. This reaction may be represented by the chemical equation 2H2+O2→2H2O.
The formula weight of hydrogen gas is 2.01594, that of oxygen gas 31.9998, and that of water 18.01534. Our chemical equation is numerically equivalent to 2×2.01594+31.9998=2×18.01534 or 4.03188+31.9998=36.03068 if the formula weight of each reactant is substituted for the formula of that reactant. From this equation we know,
for example, that 4.03188 grams of hydrogen gas will react with 31.9998 grams of oxygen gas to yield 36.03068 grams of water. The relative proportions by weight of these reactants is the same in any
reaction of hydrogen and oxygen to form water. These relative weights computed from the chemical equation are sometimes called equation weights.

calculations
As you will soon see in the technique section of this laboratory,
this experiment requires that you weigh a certain mass of the unknown salt, dissolve it into a solvent, and pass it over a column of ion-exchange resin. As mentioned previously, this process serves the purpose of converting the salt into its corresponding acid. For example, KCl, LiCl, and NaCl would all be transformed to HCl. Once the salt has been converted, the acid solution which is a result of this procedure is titrated using a standardized solution of sodium hydroxide. With the information obtained from your titration, you can calculate the number of moles of NaOH used.
-
- moles of NaOH = (L of NaOH) (M of NaOH)
- The number of moles of NaOH used is equal to the number of moles of H+ present in the solution, which is equal to the number of moles of the unknown salt.
- moles of NaOH = moles of H+ = moles of salt
- Since the molecular weight is equal to grams per mole:
- MWT = g/mol,
- and the number of grams and the number of moles of salt are known, you can determine the molecular weight of the compound and its identity.
Weight Formula Derivation
A fact that is well known by experiment is that a freely falling body irrespective of the mass would experience acceleration. This acceleration is due to the influence of gravity and its denotation is by ‘g’. Furthermore, this acceleration acts towards the center of the Earth. Therefore, from the Second Law of Motion we certainly have:
F = ma
Here, F = force, m = mass, and a = acceleration.
Now for freely falling bodies
a = g
F = w
Here, g = gravity and w = weight
Hence, w = mg
From Empirical Formula to Molecular Formula
The empirical formula gives only the relative numbers of atoms in a substance in the smallest possible ratio. For a covalent substance, chemists are usually more interested in the molecular formula, which gives the actual number of atoms of each kind present per molecule. Without additional information, however, it is impossible to know whether the formula of penicillin G, for example, is C16H17N2NaO4S or an integral multiple, such as C32H34N4Na2O8S2, C48H51N6Na3O12S3, or (C16H17N2NaO4S)n, where n is an integer.
Consider glucose, the sugar that circulates in our blood to provide fuel for the body and brain. Results from combustion analysis of glucose report that glucose contains 39.68% carbon and 6.58% hydrogen. Because combustion occurs in the presence of oxygen, it is impossible to directly determine the percentage of oxygen in a compound by using combustion analysis; other more complex methods are necessary. Assuming that the remaining percentage is due to oxygen, then glucose would contain 53.79% oxygen. A 100.0 g sample of glucose would therefore contain 39.68 g of carbon, 6.58 g of hydrogen, and 53.79 g of oxygen.


To calculate the number of moles of each element in the 100.0 g sample, divide the mass of each element by its molar mass:
Once again, the subscripts of the elements in the empirical formula are found by dividing the number of moles of each element by the number of moles of the element present in the smallest amount:
The oxygen:carbon ratio is 1.018, or approximately 1, and the hydrogen:carbon ratio is approximately 2. The empirical formula of glucose is therefore CH2O,
but what is its molecular formula?
Many known compounds have the empirical formula CH2O, including formaldehyde, which is used to preserve biological specimens and has properties that are very different from the sugar circulating in the blood. At this point, it cannot be known whether glucose is CH2O, C2H4O2, or any other (CH2O)n. However, the experimentally determined molar mass of glucose (180 g/mol) can be used to resolve this dilemma.
First, calculate the formula mass, the molar mass of the formula unit, which is the sum of the atomic masses of the elements in the empirical formula multiplied by their respective subscripts. For glucose,
This is much smaller than the observed molar mass of 180 g/mol.
Second
determine the number of formula units per mole. For glucose, calculate the number of (CH2O) units
—that is, the n in (CH2O)n—by dividing the molar mass of glucose by the formula mass of CH2O:
Each glucose contains six CH2O formula units, which gives a molecular formula for glucose of
(CH2O)6, which is more commonly written as C6H12O6. The molecular structures of formaldehyde and glucose, both of which have the empirical formula CH2O, are shown in Figure 3.2.4.
MORE POSTS:




