how do minerals form by evaporation?
Welcom to solsarin site ,Keep reading and find the answer about “how do minerals form by evaporation?”.
Stay with us.
Thank you for your support.


Today on this post , we’ll go through the process of formation minerals by evaporation.
(Mineral formation process )
Minerals form under an enormous range of geologic conditions. There are probably more ways to form minerals than there are types of minerals themselves. Minerals can form from volcanic gases, sediment formation, oxidation, crystallization from magma, or deposition from a saline fluid, to list a few. Some of these methods of mineral formation will be discussed below.
3 ways of formation of minerals
- Minerals form as magma or lava cools.
- Minerals form when they precipitate from hot fluids that have cooled down.
- Minerals form from dissolved substances when water evaporates.
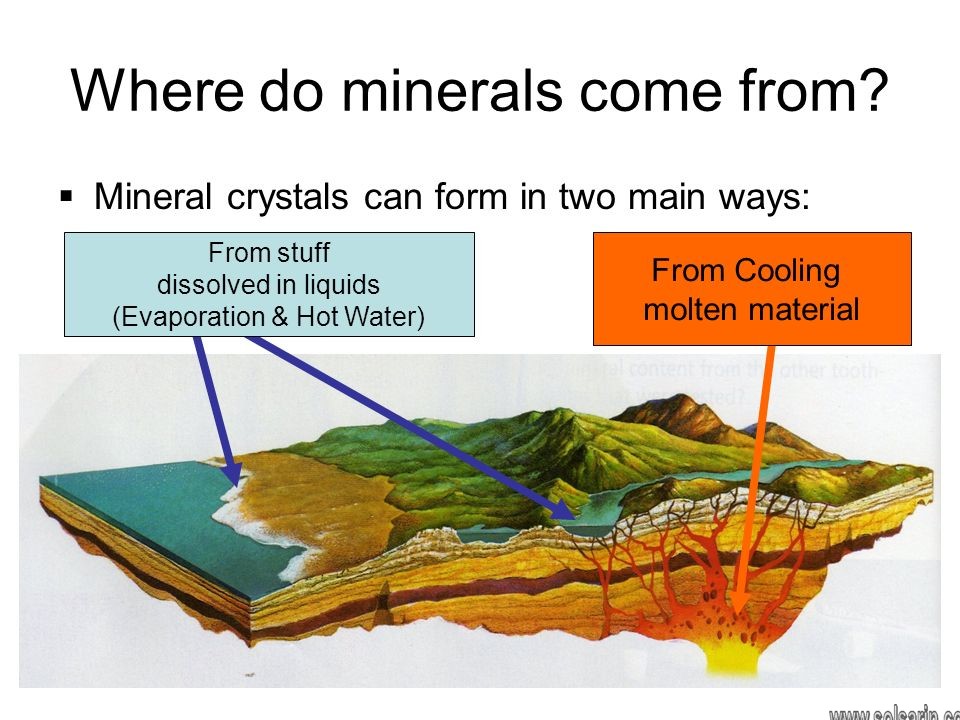

Formation from Hot Material
A rock is a collection of minerals. Imagine a rock that becomes so hot it melts. Many minerals start out in liquids that are hot enough to melt rocks. Magma is melted rock inside Earth, a molten mixture of substances that can be hotter than 1,000oC. Magma cools slowly inside Earth, which gives mineral crystals time to grow large enough to be seen clearly
The formation process of granite
Granite is rock that forms from slowly cooled magma, containing the minerals quartz (clear), plagioclase feldspar (shiny white), potassium feldspar (pink), and biotite (black).
When magma erupts onto Earth’s surface, it is called lava. Lava cools much more rapidly than magma when it is below the surface. In a cooling lava, mineral crystals do not have time to form and are very small. The chemical composition will be the same as if the magma cooled slowly.
Formation from Solutions
Water on Earth, such as the water in the oceans, contains chemical elements mixed into a solution. Various processes can cause these elements to combine to form solid mineral deposits.


Minerals from Salt Water
Fresh water contains a small amount of dissolved elements. Salt water contains many more dissolved elements. Water can only hold a certain amount of dissolved substances. When the water evaporates, it leaves behind a solid layer of minerals (Figure below). At this time, the particles come together to form minerals. These solids sink to the bottom. The amount of mineral formed is the same as the amount dissolved in the water. Seawater is salty enough for minerals to precipitate as solids. Some lakes, such as Mono Lake in California, or Utah’s Great Salt Lake, can also precipitate salts.
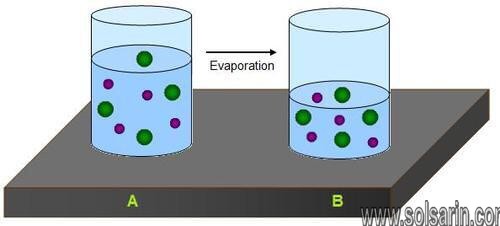

When the water in glass A evaporates, the dissolved mineral particles are left behind.
Salt easily precipitates out of water, as does calcite (Figure below). The limestone towers pictured below are made mostly of the mineral calcite. The calcite was deposited in the salty and alkaline water of Mono Lake, in California. Calcium-rich spring water enters the bottom of the lake. The water bubbles up into the alkaline lake. The calcite “tufa” towers form. When the lake level drops, the tufa towers are revealed.
(Minerals from Hot Underground Water)
Underground water can be heated by magma. The hot water moves through cracks below Earth’s surface. Hot water can hold more dissolved particles than cold water. The hot, salty solution has chemical reactions with the rocks around it.
The water picks up more dissolved particles. As it flows through open spaces in rocks, the water deposits solid minerals. When a mineral fills cracks in rocks, the deposits are called veins. Pictured below is a white quartz vein .
When the minerals are deposited in open spaces, large crystals grow. These rocks are called geodes. Pictured below is a geode that was formed when amethyst crystals grew in an open space in a rock .
What is evaporation?
the process of turning from liquid into vapour.
What is evaporite ?
evaporite, any of a variety of individual minerals found in the sedimentary deposit of soluble salts that results from the evaporation of water.
Evaporites
Evaporites are layered crystalline sedimentary rocks that form from brines generated in areas where the amount of water lost by evaporation exceeds the total amount of water from rainfall and influx via rivers and streams.
The mineralogy of evaporite rocks is complex, with almost 100 varieties possible, but less than a dozen species are volumetrically important.
Minerals in evaporite rocks include carbonates (especially calcite, dolomite, magnesite, and aragonite), sulfates (anhydrite and gypsum), and chlorides (particularly halite, sylvite, and carnallite), as well as various borates, silicates, nitrates, and sulfocarbonates.
Evaporite deposits occur in both marine and nonmarine sedimentary successions.
A closer look into modern evaporites
Though restricted in area, modern evaporites contribute to genetic models for explaining ancient evaporite deposits.
Modern evaporites are limited to arid regions (those of high temperature and low rates of precipitation), for example, on the floors of semidry ephemeral playa lakes in the Great Basin of Nevada and California, across the coastal salt flats (sabkhas) of the Middle East, and in salt pans, estuaries, and lagoons around the Gulf of Suez.
Ancient evaporates occur widely in the Phanerozoic geologic record, particularly in those of Cambrian (from 570 to 505 million years ago), Permian (from 286 to 245 million years ago), and Triassic (from 245 to 208 million years ago) age, but are rare in sedimentary sequences of Precambrian age.
They tend to be closely associated with shallow marine shelf carbonates and fine (typically rich in iron oxide) mudrocks.
Because evaporite sedimentation requires a specific climate and basin setting, their presence in time and space clearly constrains inferences of paleoclimatology and paleogeography.
Evaporite beds tend to concentrate and facilitate major thrust fault horizons, so their presence is of particular interest to structural geologists. Evaporites also have economic significance as a source of salts and fertilizer.
Evaporite deposits(how do minerals form by evaporation?)
All evaporite deposits result from the precipitation of brines generated by evaporation.
Laboratory experiments can accurately trace the evolution of brines as various evaporite minerals crystallize. Normal seawater has a salinity of 3.5 percent (or 35,000 parts per million), with the most important dissolved constituents being sodium and chlorine.
When seawater volume is reduced to one-fifth of the original, evaporite precipitation commences in an orderly fashion, with the more insoluble components (gypsum and anhydrite) forming first. When the solution reaches one-tenth the volume of the original, more soluble minerals like sylvite and halite form.
Natural evaporite sequences show vertical changes in mineralogy that crudely correspond to the orderly appearance of mineralogy as a function of solubility but are less systematic.
Nonmarine environment
Evaporite deposition in the nonmarine environment occurs in closed lakes—i.e., those without outlet—in arid and semiarid regions.
Such lakes form in closed interior basins or shallow depressions on land where drainage is internal and runoff does not reach the sea. If water depths are shallow or, more typically, somewhat ephemeral, the term playa or playa lake is commonly used.
The water flow and evaporation
Water inflow into closed lakes consists principally of precipitation and surface runoff, both of which are small in amount and variable in occurrence in arid regions.
Groundwater flow and discharge from springs may provide additional water input, but evaporation rates are always in excess of precipitation and surface runoff.
Sporadic or seasonal storms may give rise to a sudden surge of water inflow. Because closed lakes lack outlets, they can respond to such circumstances only by deepening and expanding. Subsequent evaporation will reduce the volume of water present to prestorm or normal amount; fluctuation of closed lake levels therefore characterizes the environment.
Relations between Water levels and evaporation
Such changing lake levels and water volumes lead to fluctuating salinity values. Variations in salinity effect equilibrium relations between the resulting brines and lead to much solution and subsequent reprecipitation of evaporites in the nonmarine environment.
As a result of these complexities as well as the distinctive nature of dissolved constituents in closed lake settings, nonmarine evaporite deposits contain many minerals that are uncommon in marine evaporites—e.g., borax, epsomite, trona, and mirabilite.
Shallow marine environment
Evaporite deposition in the shallow marine environment (sometimes termed the salina) occurs in desert coastal areas, particularly along the margins of such semi-restricted water bodies as the Red Sea, Persian Gulf, and Gulf of California.
Restriction is, in general, one of the critical requirements for evaporite deposition, because free and unlimited mixing with the open sea would allow the bodies of water to easily overcome the high evaporation rates of arid areas and dilute these waters to near-normal salinity.
This semi-restriction cannot, in fact, prevent a large amount of dilution by mixing; coastal physiography is the principal factor involved in brine production.
Shallow-water evaporites, almost exclusively gypsum, anhydrite, and halite, typically interfinger with tidal flat limestone and dolomite and fine-grained mudrock.
more to read on solsarin:



