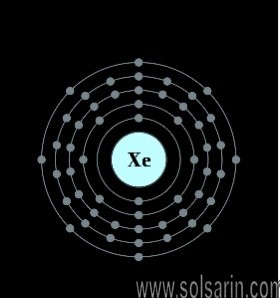
how many electrons does xenon have
Welcom to solsarin site ,Keep reading and find the answer about “how many electrons does xenon have”.
Stay with us.
Thank you for your support.
How many electron does xenon have?
54 electrons
Xenon atoms have 54 electrons and the shell structure is 2.8. 18.18. 8.
How many valence electrons does xenon have?
eight valence electrons
Xenon right over here. It is a noble gas. It has eight valence electrons. One, two, three, four, five, six, seven, eight in that fifth shell.

How many electrons does xenon give away?
Xenon has five empty but available 5 d orbitals.
Thus, highly electronegative elements like fluorine can remove an electron from the valence orbitals of xenon
to form an electron pair bond with it.
The 8 electrons are far out.
How many neutrons does xenon have?
54
The number of neutrons of xenon Xe is calculated as shown below. Therefore,
the number of neutrons in xenon (Xe) is 54.
Note: In the nucleus,
the number of electrons is the same as the number of protons for an atom.
How many protons does xenon have?
This element’s mass comes from its nucleus,
which contains 54 protons and a varying (but similar) number of neutrons.
Xenon has 17 naturally-occurring isotopes (the most for any element),
eight of which are stable, the most for any element, except tin, which has ten.
Does Xe have 18 valence electrons?
Xenon has eight valence electrons,
which are the electrons in its outer shell.
How do you find the valence electrons of xenon?
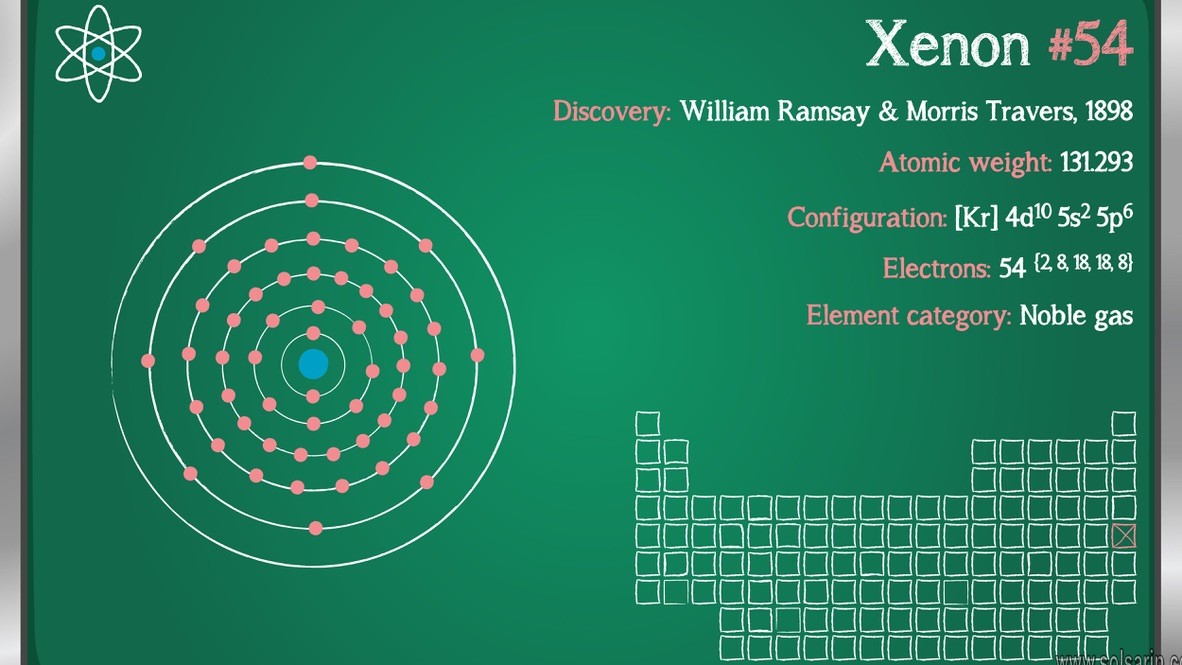
The atomic number of xenon is 54.
The electronic configuration of xenon is $[Kr]4{d^{10}}5{s^2}5{p^6}$. It has 8 valence electrons in its outermost electronic configuration so it can share its 8 electrons with other atoms.
Does xenon give or take electrons?
Xe is a large atom.
Its electrons are far from the nucleus and are less tightly held.
The ionization energy of Xe is 1170 kJ/mol.
So a xenon atom can give up some control of its electrons to a highly electronegative fluorine atom and form XeF₄.
Does xenon gain or lose electrons?
The full valence electron shells of these atoms make noble gases extremely stable and unlikely to
form chemical bonds because they have little tendency to gain or lose electrons.
Is xenon A element?
xenon (Xe), chemical element,
a heavy and extremely rare gas of Group 18 (noble gases) of the periodic table.
It was the first noble gas found to form true chemical compounds. More than 4.5 times heavier than air,
xenon is colourless, odourless, and tasteless.


How do you find the electrons?
To calculate the numbers of subatomic particles in an atom,
use its atomic number and mass number:
number of protons = atomic number.
number of electrons = atomic number.
How many protons and electrons does xenon have?
The nucleus consists of 54 protons (red) and 78 neutrons (yellow). 54 electrons (white) bind to the nucleus,
filling the outer (fifth) electron shell in what is a very stable configuration.
The stability of an element’s outer electrons determines its chemical and physical properties.
How many protons and electrons does xenon have?
The nucleus consists of 54 protons (red) and 78 neutrons (yellow). 54 electrons (white) bind to the nucleus,
filling the outer (fifth) electron shell in what is a very stable configuration.
The stability of an element’s outer electrons determines its chemical and physical properties.
How many subatomic particles does xenon have?
Now we have elemental xenon,
and thus we KNOW that the nucleus contains 54 protons .
If it didn’t possess 54 protons it would not be elemental xenon
How many protons neutrons and electrons are present in an atom of xenon XE with a mass number of 131?
The total mass number is 131.
The remaining mass must come from neutrons;
thus there are 78 neutrons.
Since the atom is neutral,
the electrons will balance the protons.
In total, there are 53 protons, 78 neutrons, and 53 electrons.
Can XE have more than 8 electrons?
Xe does not follow the octet rule.
It actually bonds. It will hold more than 8 electrons.
Xenon having valence electrons in the 4th energy level,
will also have access to the 4d sublevel,
thus allowing for more than 8 electrons.


How many valence electrons does radon have?
eight valence electrons
Radon (atomic number 86) is a noble gas like helium,
neon and argon
that is relatively unreactive because of its electron configuration,
where the octet rule is fulfilled (the atom has eight valence electrons).
How many total electrons does element 15 have?
How many total and valence electrons are in a neutral phosphorus atom? A neutral phosphorus atom has 15 total electrons.
Two electrons can go into first shell, eight in the second shell,
and it has five more in the third shell.
The third shell is the outer valence shell,
so it has 5 valence electrons.
How many electrons does radon 222 have?
The nucleus consists of 86 protons (red) and 136 neutrons (orange). 86 electrons (white) successively occupy available electron shells (rings).
Radon is a noble gas in group 18, period 6,
and the p-block of the periodic table.
How many neutrons does radon have?
136 neutrons
The chemical symbol for radon is Rn,
and the mass number is usually placed either after the symbol (Rn-222) or to the left and above it (222Rn).
In either case,
it simply designates the element radon,
which always has 86 protons,
and that the particular isotope of radon in question is the one with 136 neutrons.
How many electrons have an ML in radon?
Radon Atomic and Orbital Properties
Radon atoms have 86 electrons and the electronic shell structure is [2, 8, 18, 32, 18, 8]
with Atomic Term Symbol (Quantum Numbers) 1S0.
How many valence electrons do potassium have?
one valence electron
K is the symbol for potassium,
and the number of valence electron can be found through its’ group on the periodic table.
Hence, it has one valence electron.
What is selenium valence electrons?
Selenium specifically has an electron configuration of 2-8-18-6.
The six electrons in the outermost shell allow selenium to have a variety of valence numbers.
Selenium compounds have been found that have valences of -2, 4, and 6.
Speaking of the number six,
selenium is found to have six naturally occurring isotopes.

How many valence electrons does sodium have?
one valence electron
A: An atom of a group 1 element such as sodium has just one valence electron.
It is “eager” to give up this electron in order to have a full outer energy level,
because this will give it the most stable arrangement of electrons.
Is xenon inert?
Xenon is one of the inert or noble gases and is odorless,
colorless, tasteless and chemically non-reactive.
While not toxic on its own,
its compounds are strong oxidizing agents that are highly toxic.
How many isotopes does xenon have?
The Xenon isotopes can be supplied in various chemical forms. Naturally occurring Xenon has nine stable isotopes.
Is xenon a cation or anion?
So, the chemical formula for xenon is Xe.
Xenon losing one or more electrons in the elemental state will look like Xen+ X e n + ,
where n is the number of electrons lost.
In the case of xenon,
various compounds are reported in which
xenon exists as a cation with various oxidation states like 0, +2, +4, etc.
Which type of ligand is Xe?
The Au−Xe bond length is 274 pm (2.74 Å).
Tetraxenonogold(II) is unusual in that it is a compound of the notoriously inert atoms xenon and gold.
It is also unusual in that it uses xenon as a transition metal ligand, and in that it contains gold in the +2 oxidation state.
How many electrons does CL have?
2: The Formation of a Chlorine Ion. On the left,
the chlorine atom has 17 electrons.
On the right, the chloride ion has 18 electrons and has a 1− charge.
What is the electron number?
The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged species. This means the number of electrons and the number of protons in an element are equal. Therefore, the number of electrons in oxygen is 8.
How many electrons does this atom have?
Electrons orbiting around the nucleus of an atom are arranged in shells — also known as “energy levels.” The first shell can hold only two electrons, while the next shell holds up to eight electrons.
How many electrons does sodium have?(how many electrons does xenon have)
We know that the atomic number of sodium is 11. This tells us that sodium has 11 protons and because it is neutral it has 11 electrons.
How do you find the molar mass of Xe?(how many electrons does xenon have)
The molar mass of xenon is 131.293 g/mol. Single atoms of xenon will have, on average, a mass of 131.293 grams. Single atoms of xenon will have, on average, a mass of 131.293 u. 1 atom of xenon (Xe) has a mass of 54 u.
What properties does xenon have?(how many electrons does xenon have)
Xenon is a rare, odorless, colourless, tasteless, chemically unreactive gas. It was regarded as completely inert until, in 1962, Neil Bartlett reported synthesis of xenon haxafluoroplatinate. In a gas filled tube xenon emits blue light when excited by electrical discharge.
What is liquid xenon?(how many electrons does xenon have)
Liquid xenon is a promising material for use as a detector in the search for dark matter. … Elastic scattering of WIMPs by the nuclei of liquid Xe will produce nuclear recoils with energy up to a few tens of keV [5]. The recoil ion produces excitation and ionization in the medium.



