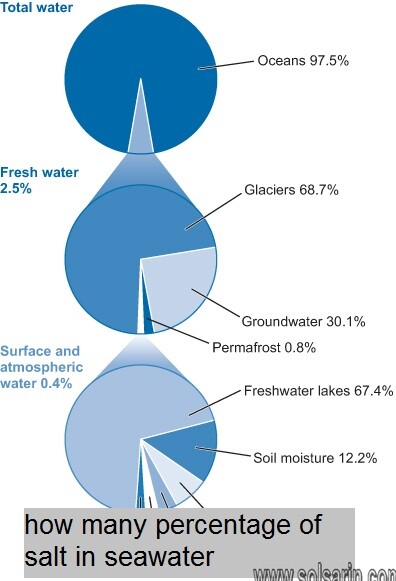
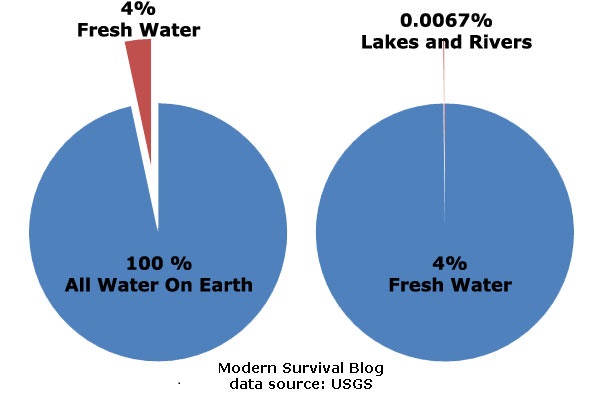
how many percentage of salt in seawater
Hello dear friends, thank you for choosing us. In this post on the solsarin site, we will talk about “ how many percentage of salt in seawater “.
Stay with us.
Thank you for your choice.
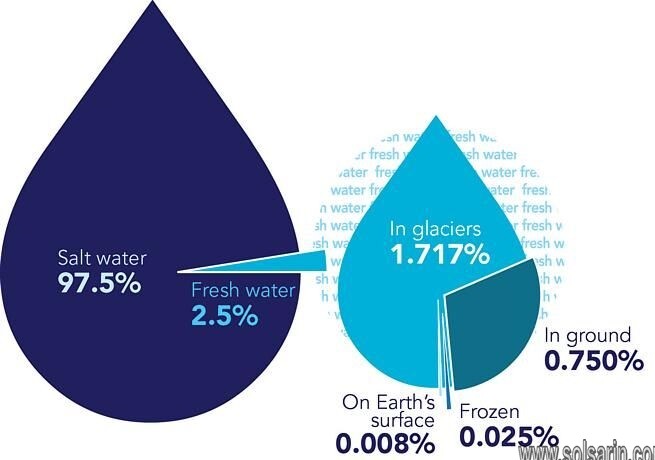

Chemical composition
Rivers add dissolved and particulate chemicals to the oceanic margins.
Organisms
Through these local and regional chemical input and removal mechanisms, each element in the oceans tends to exhibit spatial and temporal concentration variations.
Physical mixing
Physical mixing in the oceans (thermohaline and wind-driven circulation) tends to homogenize the chemical composition of seawater.
The opposing influences of physical mixing and of biogeochemical input and removal mechanisms result in a substantial variety of chemical distributions in the oceans.
Dissolved organic substances
Processes involving dissolved and particulate organic carbon are of central importance in shaping the chemical character of seawater.
rain
The “rain” of organic-rich particulate materials, resulting directly and indirectly from photosynthetic production, is a principal factor behind the distributions of many organic and inorganic substances in the oceans.
Estimates of DOC appropriate to the surface of the open ocean range between roughly 100 and 500 micromoles of carbon per kilogram of seawater
The relative abundances of reactive organic
The influence of dissolved organic
The influence of dissolved organic matter on ocean chemistry is often out of proportion to its oceanic abundance.
Salinity distribution
his uniformity of salt content results in oceans in which the salinity varies little over space or time.
Temperature distribution
Mid-ocean surface temperatures vary with latitude in response to the balance between incoming solar radiation and outgoing longwave radiation.
Superimposed on this radiation balance
Superimposed on this radiation balance are seasonal changes in the intensity of solar radiation and the duration of daylight hours .
due to the tilt of Earth’s axis to the plane of the ecliptic and the rotation of the planet about this axis.
water
This heat transfer reduces the actual change in ocean surface temperatures over the annual cycle.
In the tropics the ocean surface is warm year-round, varying seasonally about 1 to 2 °C (1.8 to 3.6 °F).
At midlatitudes the mid-ocean temperatures vary about 8 °C (14.4 °F) over the year.
At the polar latitudes the surface temperature remains near the freezing point of seawater, about −1.9 °C (28.6 °F).
isolation of water from the open ocean, and processes that control stability of the surface water combine to increase the annual range of nearshore ocean surface temperature.
Thermal properties
The unit of heat called the gram calorie is defined as the amount of heat required to raise the temperature of one gram of water 1 °C.
The kilocalorie, or food calorie, is the amount of heat required to raise one kilogram of water 1 °C.
Heat capacity is the amount of heat required to raise one gram of material 1 °C under constant pressure.
In the International System of Units (SI), the heat capacity of water is one kilocalorie per kilogram per degree Celsius.
Water has the highest heat capacity of all common Earth materials.
The heat capacity of any material
The heat capacity of any material can be divided by the heat capacity of water to give a ratio known as the specific heat of the material.
Seawater of 35 psu has a specific heat of 0.932 compared with 1.000 for pure water.
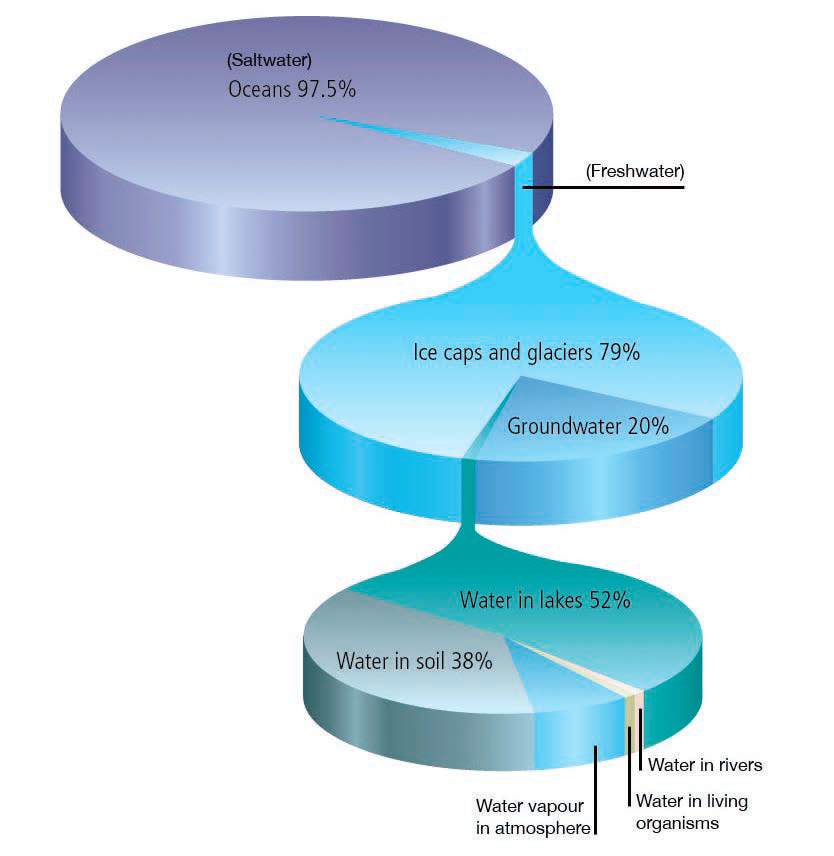

Pure water
Pure water freezes at 0 °C and boils at 100 °C (212 °F) under normal pressure conditions.
When salt is added, the freezing point is lowered and the boiling point is raised. The addition of salt also lowers the temperature of maximum density below that of pure water (4 °C [39.2 °F]).
Density of seawater and pressure
The density of seawater is a function of temperature, salinity, and pressure.
These two factors led oceanographers to adopt a density unit called sigma-t (σt
The σt has no units and is an abbreviated density of seawater controlled by salinity and temperature only.
The σt of seawater increases with increasing salinity and decreasing temperature.