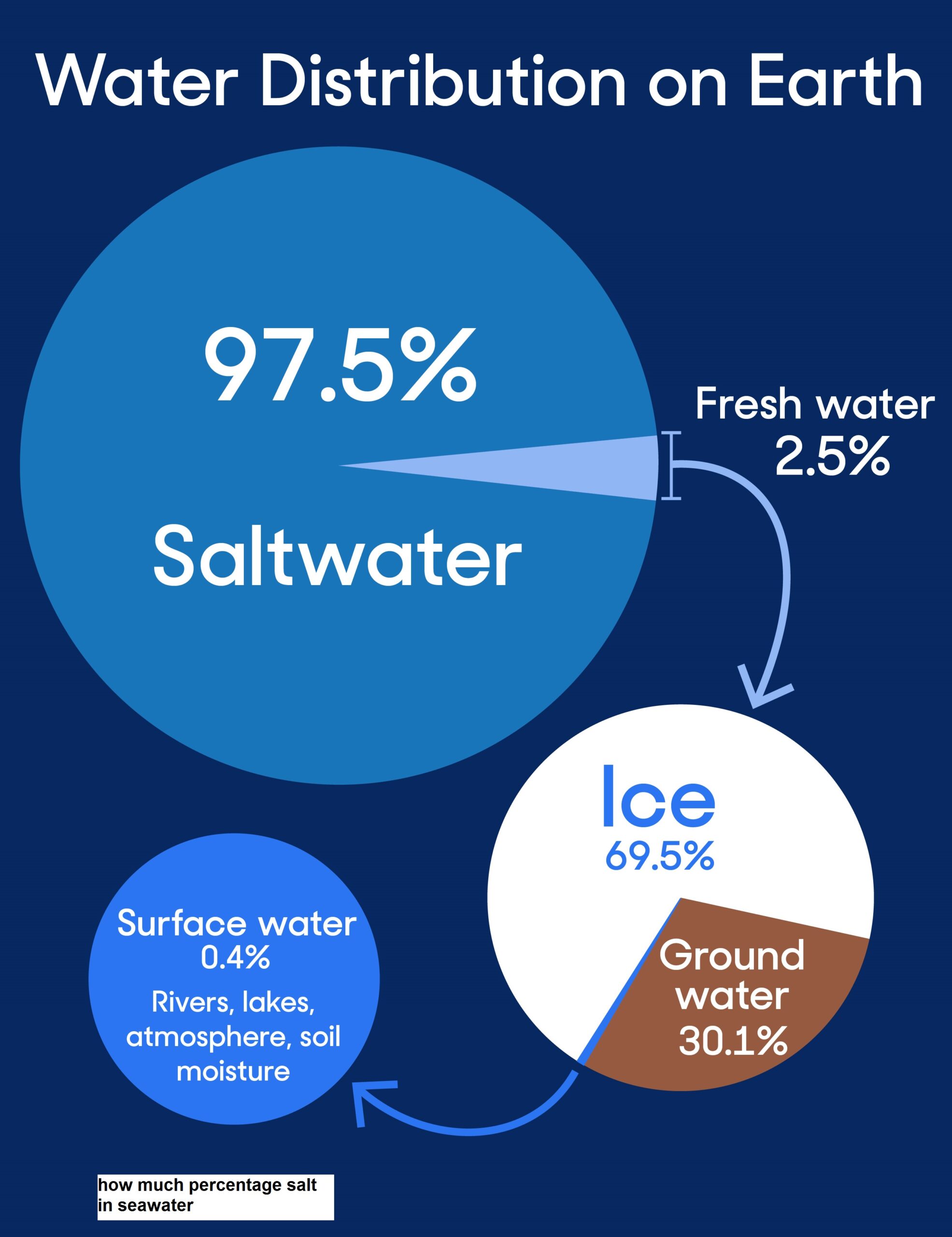
how much percentage salt in seawater
Hello dear friends, thank you for choosing us. In this post on the solsarin site, we will talk about “ how much percentage salt in seawater “.
Stay with us.
Thank you for your choice.

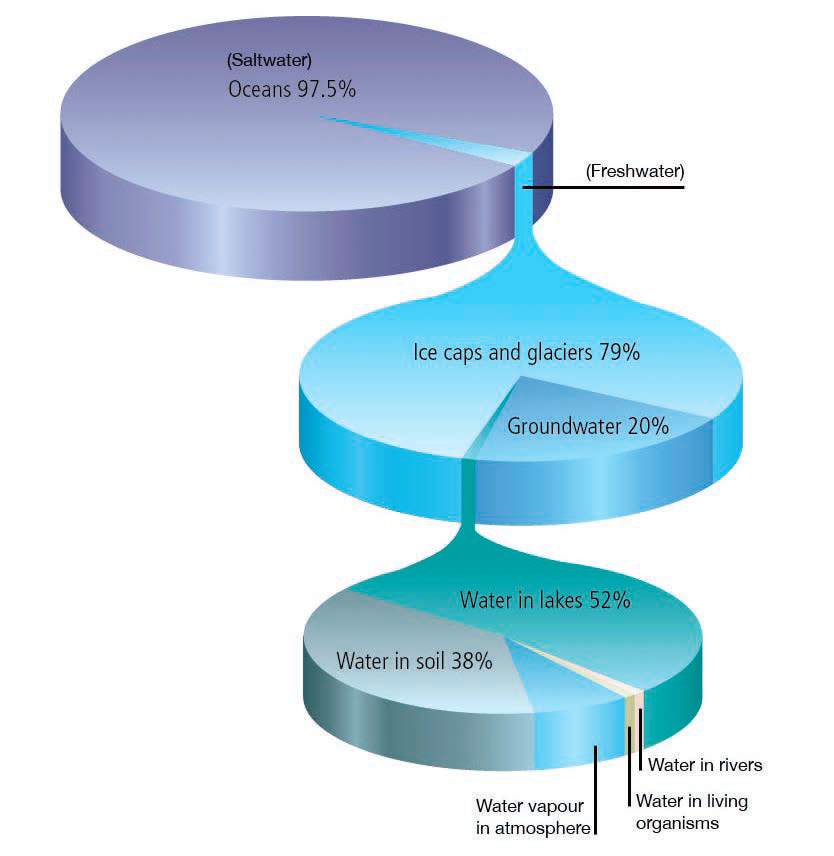
Why is the ocean salty?
Salt in the ocean comes from two sources: runoff from the land and openings in the seafloor.
Rocks on land are the major source of salts dissolved in seawater. Rainwater that falls on land is slightly acidic, so it erodes rocks. This releases ions that are carried away to streams and rivers that eventually feed into the ocean. Many of the dissolved ions are used by organisms in the ocean and are removed from the water. Others are not removed, so their concentrations increase over time.
Another source of salts in the ocean
Another source of salts in the ocean is hydrothermal fluids, which come from vents in the seafloor. Ocean water seeps into cracks in the seafloor and is heated by magma from the Earth’s core. The heat causes a series of chemical reactions. The water tends to lose oxygen, magnesium, and sulfates, and pick up metals such as iron, zinc, and copper from surrounding rocks. The heated water is released through vents in the seafloor, carrying the metals with it. Some ocean salts come from underwater volcanic eruptions, which directly release minerals into the ocean.
Salt domes
Salt domes also contribute to the ocean’s saltiness. These domes, vast deposits of salt that form over geological timescales, are found underground and undersea around the world. They are common across the continental shelf of the northwestern Gulf of Mexico.

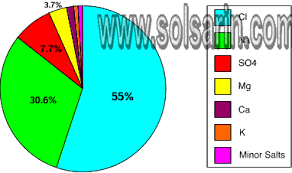
chloride and sodium
Two of the most prevalent ions in seawater are chloride and sodium. Together, they make up around 85 percent of all dissolved ions in the ocean. Magnesium and sulfate make up another 10 percent of the total. Other ions are found in very small concentrations. The concentration of salt in seawater (salinity) varies with temperature, evaporation, and precipitation. Salinity is generally low at the equator and at the poles, and high at mid-latitudes. The average salinity is about 35 parts per thousand. Stated in another way, about 3.5 percent of the weight of seawater comes from the dissolved salts.
The chemical composition of seawater is influenced by a wide variety of chemical transport mechanisms. Rivers add dissolved and particulate chemicals to the oceanic margins.
Wind-borne particulates are carried to mid-ocean regions thousands of kilometres from their continental source areas. Hydrothermal solutions that have circulated through crustal materials beneath the seafloor add both dissolved and particulate materials to the deep ocean.
Organisms in the upper ocean convert dissolved materials to solids, which eventually settle to greater oceanic depths.
Particulates in transit to the seafloor
Particulates in transit to the seafloor, as well as materials both on and within the seafloor, undergo chemical exchange with surrounding solutions.
Through these local and regional chemical input and removal mechanisms, each element in the oceans tends to exhibit spatial and temporal concentration variations.
Physical mixing in the oceans (thermohaline and wind-driven circulation) tends to homogenize the chemical composition of seawater.
The opposing influences of physical mixing and of biogeochemical input and removal mechanisms result in a substantial variety of chemical distributions in the oceans.
Seawater
is water from a sea or ocean. On average, seawater in the world’s oceans has a salinity of about 3.5% (35 g/l, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na+
) and chloride (Cl−
) ions). Average density at the surface is 1.025 kg/l. Seawater is denser than both fresh water and pure water (density 1.0 kg/l at 4 °C (39 °F)) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases.
At typical salinity, it freezes at about −2 °C (28 °F).The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F).[2] Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.
resource: wikipedia

read more: