hydrogen how to find mass percent of hydrogen in water
Hello welcome to the solsarin site. stay with us to find out “how to find mass percent of hydrogen in water”
What is the Mass Percent of Hydrogen in Water?
Molar Mass of Elements
For any compound, you determine the total molar mass by adding up the molar masses of each element. When you look up an element in the periodic table, the number on top is the atomic number, and the one beneath the element symbol is the average atomic mass, given in atomic mass units (amu). For any atoms that appear more than once in the molecule, multiply the molar mass by the element’s quantity in the chemical formula. For example, there are two hydrogen atoms in the water molecule, so multiply hydrogen’s atomic mass by 2.
Molar Mass of Water
The atomic mass of hydrogen taken from the periodic table is 1.008. Because the molecule has two hydrogen atoms, multiply 1.008 by 2 to get 2.016. The atomic mass of oxygen is 16.00, and the molecule has only one oxygen atom, so oxygen’s total mass remains 16.00. Add 2.016 to 16.00 to get 18.016. This is the total molar mass of water.
Mass Percent of Hydrogen
To find the mass percent of hydrogen in water, take the molar mass of hydrogen in the water molecule, divide by the total molar mass of water, and multiply by 100. Dividing 2.016 by 18.016 gives you 0.1119. Multiply 0.1119 by 100 to get the answer: 11.19 percent.
Mass Percent of Oxygen
You can use two methods to find the mass percent of oxygen in water. From the calculation above, you know the percentage of hydrogen is 11.19 percent, and water has only hydrogen and oxygen, so the two added together must equal 100 percent. Subtract 11.19 from 100 to get 88.81 percent. The second method is the same as for finding the mass percent of hydrogen. From previous calculations, you know the total molar mass of oxygen in water is 16.00. Divide 16.00 by the total molar mass of water, 18.016, to get 0.8881. Multiply by 0.8881 by 100 to get the percentage: 88.81 percent.
Ratios of Mass
As the water molecule has exactly two elements, you can use the numbers already calculated to determine the ratios of mass. For example, to find the ratio of mass of hydrogen to oxygen in water, divide the total molar mass of hydrogen, 2.016, by the molar mass of oxygen, 16.00 and get 0.126. To find the ratio of oxygen to hydrogen, divide 16.00 by 2.016 and get 7.937. This means in water, oxygen outweighs hydrogen by almost 8 to 1.
How to Calculate Theoretical Percent
Each chemical compound contains a combination of atoms, and one way to understand theoretical percent is to equate it to the percentage of a particular element in a compound. This percentage isn’t based on numbers of atoms, but on the total mass of the element relative to the mass of the compound.
Another way to understand theoretical percent is in the context of a chemical reaction. In any reaction, the total molar mass of each element involved in the reaction must be conserved. You can calculate the mass of each product as long as you know the chemical formulas of all the reactants and products. This is the theoretical yield for that product. The actual yield is almost always less for a number of reasons. The ratio of actual to theoretical yield gives you a quantity called percent yield.
TL;DR (Too Long; Didn’t Read)
To calculate the theoretical percentage of an element in a compound, divide the molar mass of the element by the mass of the compound and multiply by 100. In a chemical reaction, the percent yield of a product is its actual yield divided by its theoretical yield and multiplied by 100.
Calculating Theoretical Percent of an Element
To calculate the theoretical percent of each element in a compound, you have to know the chemical formula of the compound. Knowing this, you can calculate the mass of the compound by looking up the atomic masses of each of the elements and adding them together.
If an element has a subscript following its symbol, multiply the mass of that element by the subscript before doing the summation.
Once you know the mass of the compound, you calculate the theoretical percent of each element by dividing the atomic mass of that element – multiplied by the subscript that follows it in the formula – by the mass of the compound and multiplying by 100.
Example: What is the theoretical percent of carbon in methane (CH4)?
Find the masses in the periodic table. The atomic mass of one mole of carbon (C) is 12.01 g, and that of hydrogen (H) is 1.01 g, rounding to two places.
Sum the masses of carbon and hydrogen. Remember to multiply the mass of hydrogen by 4 because there are four hydrogen atoms in the molecule, indicated by the subscript. This gives a mass of 16.05 g for the methane molecule.
Divide the mass of carbon by the mass of methane and multiply by 100.
(12.01 ÷ 16.05) × 100 = 74.83%
Note that, even though methane contains four hydrogen atoms and only one carbon atom, carbon makes up three-quarters of the compound.

Calculating Percent Yield in a Reaction
You calculate the theoretical yield of a particular product in a reaction from the balanced equation for the reaction, and you determine the actual yield by experiment. There’s no way to predict actual yield – you have to measure it. The percent yield is the actual yield divided by the theoretical yield multiplied by 100.
Example: Calcium carbonate (CaCO3) dissolves in water to produce calcium bicarbonate (CaO) and carbon dioxide (CO2). If 16 g of CaCO3 yields 7.54 g CaO, what is the percent yield of CaO?
The balanced equation for the reaction is: CaCO3 –> CaO + CO2.
Divide the measured mass of calcium carbonate (16 g) by the molar mass of the compound (100 g) to get 16 ÷ 100 = 0.16 moles.
According to the equation, one mole of CaCO3 produces one mole of CaO, so 0.16 moles of CaCO3 produces 0.16 moles of CaO. The molar mass of CaO is 56 g, so 0.16 moles of the compound = 56 g × 0.16 = 8.96 g.
In this experiment, only 7.54 g of CaO were recovered, so the percent yield is:
(7.54 ÷ 8.96) × 100 = 84.15%
How to Find Mass Percentage
calculate the total mass of the solution. In the example, the solution mass is equal to mass (NaCl) + mass (NaHCO3) + mass (water) = 10 g + 6 g + 120 g = 136 g.
Divide the mass of the first dissolved component by the solution mass, and then multiply the result by 100 to calculate the mass percentage. In our example, the first dissolved compound is NaCl; the mass percent is (10 g / 136 g) x 100 percent = 7.35 percent.
Divide the mass of the second dissolved component by mass of the solution followed by multiplying by 100 to calculate the mass percentage. In this example, the second dissolved compound is NaHCO3, and its mass percentage is (6 g / 136 g) x 100 percent = 4.41 percent.
How to Convert From Moles Per Liter to Percentage
Concentration represents the amount of the compound dissolved in the solution.
Molarity is the number of moles of a substance in 1 liter of the solution. Another unit of the concentration, weight percentage, refers to the ratio of the mass of the solute (a dissolved substance) to the mass of the solution. Converting between concentrations is frequently required for various problems in chemistry.
Determine atomic masses of elements that comprise the dissolved compound using the Periodic Table of the Elements. For example, if the compound in the solution is potassium chloride (KCl), the atomic mass of potassium (K) is 39 and that of chlorine (Cl) is 35.5.
Multiply the atomic mass by the number of the respective atoms in the molecule, and then sum up the products to calculate the molar mass In this example, the molar mass of KCl is 39 x 1 + 35.5 x 1 = 74.5.
Multiply the molar mass of the compound by the molarity to calculate the amount of the dissolved substance in one liter of the solution. For example, 0.5 M of KCl solution contains 74.5 x 0.5 = 37.25 g of the salt.
Multiply the density of the solution by 1,000 ml (1 liter) to calculate the mass of the 1L of the solution. For example, if the density of 0.5 M KCl solution is 1.1 g/ml, the weight of 1 liter of the solution is 1.1 x 1,000 = 1,100 g.
Divide the mass of the dissolved compound by the mass of the solution, and multiply the result by 100 to calculate percentage. In this example, the solution of KCl is (37.25 ÷ 1,100) x 100 = 3.39 percent.
Conversion of PPM to Micromoles
Parts per million (ppm) is a unit of concentration.
When concentration of a substance is low, such as water contaminated with certain metals (iron, cadmium or magnesium), ppm becomes more convenient than standard units of concentration – molarity or weight percent – used in chemistry. A mole is the unit in chemistry that measures the amount of substance.
To make basic stoichiometric chemical calculations you need to convert ppm to moles or micromoles.
Multiply ppm by the weight of the solution, then divide by 1,000,000 to compute the mass of the compound. For example, if ppm of cadmium (Cd) is 20 and the mass of the solution is 500 grams, then the mass of the dissolved cadmium is (20 x 500) /1,000,000 = 0.01 grams.
Get the atomic mass of the element presented in water from the Periodic Table of the Elements. In this example, the atomic mass of cadmium (Cd) is 112.
Divide the weight of the compound by the atomic mass to calculate the number of moles. In this example, the number of moles is 0.01 / 112 = 0.000089 moles.
Multiply the number of moles by 1,000,000 to calculate micromoles. In this example 0.000089 x 1,000,000 = 89 micromoles.


Random Posts
How to Calculate Mass Ratio
In chemistry, mass ratio, often called “percent composition by mass,” is the proportion of a particular molecule that consists of each that molecule’s constituent elements.
For example, water consists of 11.1 percent hydrogen (H) and 88.9 percent oxygen (O), meaning that a 1,000-gram sample of water (equal to 1 liter in volume) consists of 111 g of H (0.111 × 1,000 = 111) and 889 g of O (0.889 × 1,000).
This principle gives rise to the Law of Constant Composition, put forth by Joseph Proust in 1800: A given compound always has the same proportion by mass of its constituent elements. For instance, water always has exactly 8 grams of oxygen for every gram of hydrogen. Carbon dioxide always has 2.67 g of oxygen for every gram of carbon.
Calculating mass ratios is easy enough if you have access to a periodic table (see Resources) and the means to do basic algebra.
Say you want to calculate the mass ratio of sulfuric acid, H2SO4.
H2SO4 contains hydrogen (H), sulfur (S) and oxygen (S). From the periodic table, you can see that the molar masses of these elements are:
H = 1.00
S = 32.06
O = 16.00
Step 2: Determine the Mass of Each Individual Element Present
In this step, you multiply the number of atoms in one molecule of the compound by the molar masses you collected in Step 1.
The number of atoms is simply the subscript after the element’s abbreviation in the molecular formula, with the omission of a subscript signifying “1.”
There are two H atoms present, one S atom and four O atoms, so you have:
H = (2)(1.00) = 2 g
S = (1)(32.06 g) = 32.06 g
O = (4)(16.00 g) = 64 g
Step 3: Determine the Molar Mass of the Compound
Add together the figures you calculated in Step 2:
2 + 32.06 + 64 = 98.06 g
Step 4: Divide the Mass of Each Element Present by the Molar Mass
This means dividing the individual masses from Step 2 by the result of Step 3.
For H, you have 2 ÷ 98.06 = 0.0204 = 2.04 percent hydrogen
For S, you have 32.06 ÷ 98.06 = 0.3269 = 32.69 percent sulfur
For O, you have 64 ÷ 98.06 = 0.6527 = 65.27 percent oxygen
Tip
To check your work, make sure your percentages sum to 100, allowing for tiny differences owing to rounding:
2.04 + 32.69 + 65.27 = 100.0


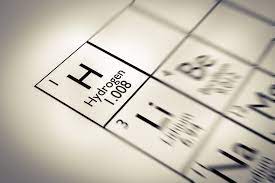
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. With a standard atomic weight of 1.008, hydrogen is the lightest element in the periodic table. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all baryonic mass.[note 1] Non-remnant stars are mainly composed of hydrogen in the plasma state. The most common isotope of hydrogen, termed protium (name rarely used, symbol 1H), has one proton and no neutrons.
read more:




