is glucose acidic basic or neutral?
Hi, welcome to solsarin site, today we want to talk about“is glucose acidic basic or neutral”,
thank you for choosing us.
is glucose acidic basic or neutral?
Glucose has neither an acidic nor a basic nature. It is considered neutral, and its pH value is 7 .It does not donate hydrogen ions on dissolving as most of the acids do. Neither does it donate hydroxyl ions like the base. Therefore, it is considered to be neutral.
Is glucose a neutral substance?
Glucose solution is neutral.
Is glucose in water an acid or base?
Glucose is neither acidic nor basic in nature. It is considered to be neutral and its pH value is also 7.
What is the pH of glucose?
As measured by the formation of 5-(hydroxymethyl)-2-furancarboxaldehyde (HMF) at 100°C, fructose is most stable between pH 4 and 6 and glucose between pH 2 and 6.

Is glucose neutral in nature?
Explanation: Glucose is a neutral sugar that doesn’t donate hydrogen ions on dissolving as acids do, nor does it donate hydroxyl ions as bases do.
Is glucose a strong base?
Glucose is more acidic than simple alcohols with a pKa of about 12 (Ka = 10-12). Compare this to methanol with a pKa of 15.5 (Ka = 10-15.5). The electronegative oxygen atoms in the molecule pull electron density away from the carbon atom bearing the negatively charged oxygen in the conjugate base, stabilizing it.
Why does glucose and alcohol do not show acidic character?
Is lemon juice a strong acid or weak acid?
Lemons are extremely acidic. Any chemical with a pH less than 7 is considered acidic. Lemon juice has a pH of around 2.0, ranging between 2 and 3. To put that in perspective, the pH of battery acid (sulfuric acid) is 1.0, while the pH of an apple is about 3.0.
Why are compounds like alcohol and glucose not acidic?
Compounds like alcohol and glucose do not dissociate in water to form hydrogen ions neither do they accept electrons. Hence, they are considered not to exhibit acidic behavior.
Which is an acid and which is a base?
Pure sugar, or glucose, is a neutral substance. A neutral substance is a substance that does not exhibit acidic or basic properties. Neutral substances like sugar do not trigger a reaction on a Litmus paper. Generally, an acid contains hydrogen, and a base contains hydroxide.

Is H2SO4 an Acid?
The “King of Chemicals” is called sulphuric acid (H2SO4) and it is used in the processing of a very significant variety of other useful chemicals, such as hydrochloric acid, nitric acid, colourants, medications, etc.
A corrosive material that is toxic to the skin, eyes, teeth, and lungs is sulfuric acid (H2SO4). Death may result from extreme exposure. The amount of radiation depends on the dosage, length and type of work conducted.
Glucose
Glucose, also known as D-glucose or dextrose, is a member of the class of compounds known as hexoses. Hexoses are monosaccharides in which the sugar unit is a is a six-carbon containing moiety. The glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form, the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. In aqueous solution, both forms are in equilibrium and at pH 7 the cyclic one is predominant. Glucose is a neutral, hydrophilic molecule that readily dissolves in water. It exists as a white crystalline powder.
Glucose

Acidity of Glucose
Glucose is more acidic than simple alcohols with a pKa of about 12 (Ka = 10-12). Compare this to methanol with a pKa of 15.5 (Ka = 10-15.5). The electronegative oxygen atoms in the molecule pull electron density away from the carbon atom bearing the negatively charged oxygen in the conjugate base, stabilizing it.
Glucose is a polyprotic acid with 5 OH groups. One of the protonation/deprotonation equilibria for linear glucose.
Deprotonation of an alcohol group in glucose can facilitate the cyclization reaction because the conjugate base (alkoxide) is a much stronger nucleophile. It more rapidly adds to the electrophilic aldehyde carbon.
Is sugar a neutral substance?
Pure sugar, or glucose, is a neutralsubstance. A neutral substance is a substancethat does not exhibit acidic or basic properties. Neutralsubstances like sugar do not trigger a reaction on aLitmus paper.
Consequently, is sugar solution neutral?
Furthermore, what pH is sugar? sugar is neutral. So pH of sugar watershould just be 7. The sweet taste is your nerve ending sensing thesugar on its receptor and is not pHrelated.
Herein, what is a neutral substance?
Neutral substance is a substance thatshows no acid or base properties, has an equal number of hydrogenand hydroxyl ions and does not change the colour oflitmus-paper.
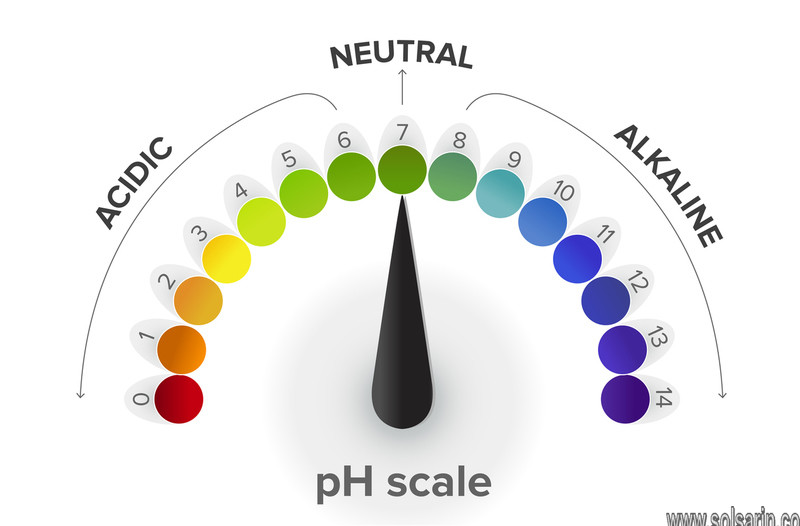
The pH of a Solution
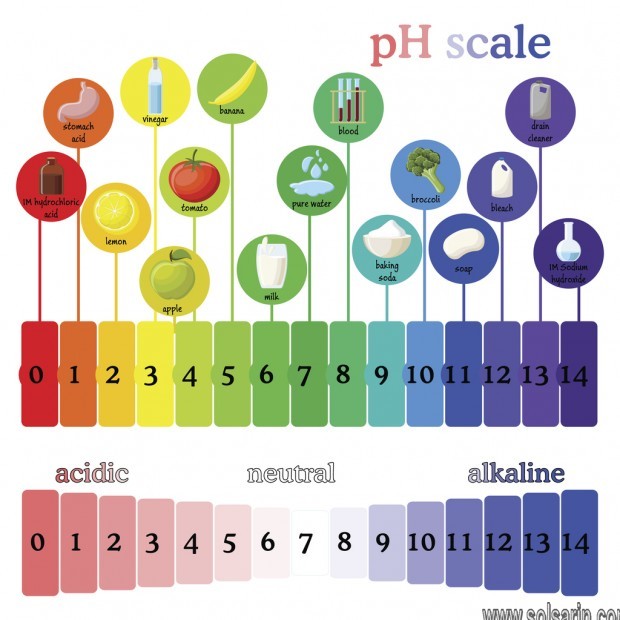
The pH level of a solution shows whether it is acidic, alkaline or neutral. Neutral means it is neither acidic nor alkaline. On a scale of 0 to 14, a pH level of 7 is neutral, a pH level lower than 7 means a solution is acidic, and a pH level greater than 7 means a solution is alkaline. Pure or distilled water has a pH level of 7.
Take the Litmus Test
If your lab has litmus paper, you can use it to determine your solution’s pH. When you place a drop of a solution on the litmus paper, the paper changes color based on the pH of the solution. Once the color changes, you can compare it to the color chart on the paper’s package to find the pH. With unknown solutions, you should wear gloves, put on eye protection and work under a fume hood to be safe.
Probe for the Answer
A pH meter will make short work of identifying the pH of your solution. These meters have a glass probe that measures a solution’s ion concentration. To use a pH meter, place a small portion of your solution in a beaker or test tube, rinse the probe of the pH meter, and then place the probe into your solution. Within seconds, the readout will tell you the pH. After taking your measurement, rinse the probe again and place it back in its storage solution.

Memorize Some Solutions
Water and blood are both neutral. Many household cleaners, such as bleach and ammonia, are basic, as is sodium hydroxide. Citric juices, coffee and wine are acidic. Solutions with the word “acid” in them, such as stomach acid and hydrochloric acid are acidic.
Adding Sugar to Other Liquids
Adding sugar toliquids besides water, such as lemonade, fruit juice or tea, will make them taste sweeter, but this has nothing to do with the pH level. it will not affect the pH level of the liquid because sugar simply does not have the chemical capacity to do this. This is an important distinction to make because many people think sugar is acidic. This is not strictly true, at least not in relation to the pH scale. However, sugar produces lactic acid when its glycoproteins attract bacteria. For example, when you drink sugary drinks, and the glycoproteins stick to your teeth. The bacteria feed on fructose and produce lactic acid, which may contribute to tooth decay.
MORE POSTS: