is milk a suspension?
Hi, welcome to solsarin site, today we want to talk about“is milk a suspension“,
thank you for choosing us.
is milk a suspension?
Milk is a colloid, with tiny globs of butterfat suspended throughout the liquid. suspension A mixture in which particles are dispersed throughout the bulk of a fluid.
Is milk a solution or a suspension?
Milk is not a solution because it has more than one phase suspended in it — it has a liquid phase and a solid phase. Unhomogenized milk is not a solution, it’s a suspension because the fat (aka cream) will separate from the rest of the milk and rise to the top, since fat is less dense than water.
Is milk a suspension or colloid?
The difference between a colloid and a suspension is that the particles will not settle to the bottom over a period of time, they will stay suspended or float. An example of a colloid is milk. Milk is a mixture of liquid butterfat globules dispersed and suspended in water.


Is milk an emulsion or suspension?
Is milk a solution or heterogeneous mixture?
Whole milk is actually a heterogeneous mixture composed of globules of fat and protein dispersed in water. Homogeneous mixuters are those in which the components are evenly distributed over the major component/constitute of the mixture.
Is milk homogeneous mixture?
Homogeneous mixtures are also called solutions. Milk, for example, appears to be homogeneous, but when examined under a microscope, it clearly consists of tiny globules of fat and protein dispersed in water. The components of heterogeneous mixtures can usually be separated by simple means.
What kind of solution is milk?
People also ask, what kind of solution is milk what happens when such a solution is filtered?
Although it appears to be homogeneous, milk is actually not a solution – it is a colloid. This is because many of the particles in milk (like the fat and protein globules) are > 1 nm. In fact, this is make evident by the fact that they settle to the bottom after a while.
Beside above, what type of colloid is milk? Milk is an emulsified colloid of liquid butterfat globules dispersed within a water-based solution.
Similarly, is milk a solution colloid or suspension?
A colloid is a mixture where very small particles of one substance are evenly distributed throughout another substance. An example of a colloid is milk. Milk is a mixture of liquid butterfat globules dispersed and suspended in water.


Why is milk suspended?
Milk is not a solution because it has more than one phase suspended in it — it has a liquid phase and a solid phase. Unhomogenized milk is not a solution, it’s a suspension because the fat (aka cream) will separate from the rest of the milk and rise to the top, since fat is less dense than water.
Is tea with milk a suspension?
Milk is not a solution because it has more than one phase suspended in it — it has a liquid phase and a solid phase. Unhomogenized milk is not a solution, it’s a suspension because the fat (aka cream) will separate from the rest of the milk and rise to the top, since fat is less dense than water.
Why does the Milk is called a Suspension?
All these characteristics are found in milk and that is why milk is a suspension.
Which is an example of a colloid or suspension?
Similarly one may ask, is milk a solution colloid or suspension? A colloid is a mixture where very small particles of one substance are evenly distributed throughout another substance. An example of a colloid is milk. Milk is a mixture of liquid butterfat globules dispersed and suspended in water.
Is there such a thing as a suspension of milk?
Is Milk a Suspension? No, milk is not a suspension. As discussed above, a suspension is a liquid with undissolved particles mixed up in it. Those undissolved particles may float around, but if you leave it alone, the particles in the water will eventually settle to the bottom of the contain, pulled down by gravity.


What are 5 examples of suspension?
Examples of Suspension
- Muddy water.
- Milk of magnesia.
- Sand particles suspended in water.
- Flour in water.
- Slaked lime for whitewashing.
- Paints in which dyes are suspended in turpentine oil.
Is milk a homogeneous mixture?
Mixtures
When matter has more than one type of element and/or compound, it is a mixture. The two types of mixtures are homogeneous and heterogeneous. Homogeneous mixtures have a uniform composition, while heterogeneous mixtures do not.
Milk that you buy in the store has a uniform composition throughout and does not separate upon standing, so it is a homogeneous mixture. Milk is homogenized to achieve the consistency. That’s why if you look at the label in the grocery store, it says homogenized milk. Raw milk that has not been homogenized is a heterogeneous mixture, because it will separate into different layers.
Is milk a homogeneous or heterogeneous mixture?
Is milk a mixture or compound?
Now as we know that we get milk from the cow and it is a mixture of water, fat and solids (in the form of milk protein and carbohydrates) which is mixed irrationally. Therefore milk is a mixture not a pure substance. Main compounds of milk are lactose and casein.
What’s a pure mixture?
In the more general sense, a pure substance is any homogeneous mixture. That is, it is matter that appears uniform in appearance and composition, no matter how small the sample size. Examples of pure substances include iron, steel, and water. Air is a homogeneous mixture that is often considered to be a pure substance.
What kind of a mixture is milk?
Colloids (heterogeneous) An example of a colloid is milk. Milk is a mixture of liquid butterfat globules dispersed and suspended in water. Colloids are generally considered heterogeneous mixtures, but have some qualities of homogeneous mixtures as well.
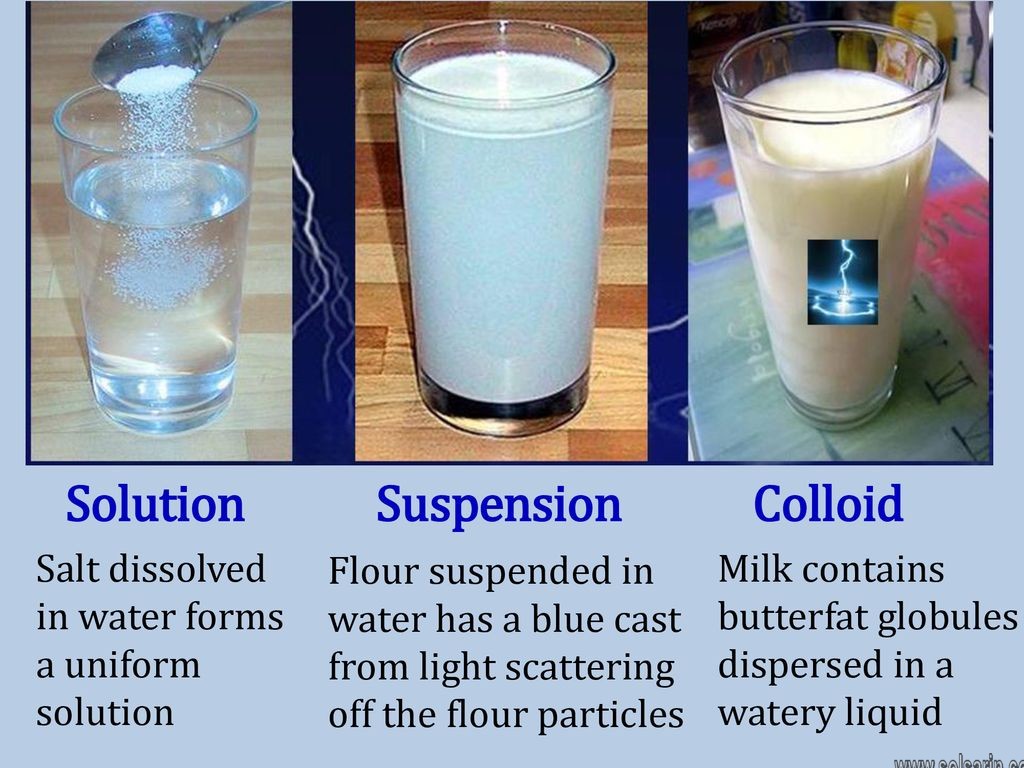

Solutions
A solution is a homogeneous mixture in which a substance dissolves (solute) in the other (solvent).
The size of particles in a solution is less than 1 nm which are not visible through naked eyes and cannot be separated easily.
The solute is said to be the dispersed phase and the solvent in which it is dissolved is the dispersion medium.
For example, a mixture of salt dissolved in water is a solution, since the particles are too small to be visible and separated easily.
Colloids
Colloids are heterogeneous mixtures that have medium-sized insoluble particles suspended in another substance.
These insoluble particles do not settle over a period of time. The particle size in colloids ranges between 1 nm and 0.1 micrometers.
These appear similar to solutions but unlike solutions, the particles are suspended rather than completely dissolved.
An example of colloids is colored gelatin, where the particles are suspended in the mixture which gives the gelatin color, while the particles are too small to settle.
You might have observed that it is very difficult to see across the smoke during a fire. The reason behind it is the same as a colloid that consists of the suspended particle.
Suspensions
A suspension is a heterogeneous mixture containing large particles that are visible and settle down over a period of time and can be filtered.
The particles are not dissolved in the mixture but rather dispersed. The particle size ranges between 1 to 50 micrometers in diameter.
A mixture of sand in water is an example of suspension, the sand is dispersed in water when mixed but settles down when untouched.




