is temperature intensive or extensive
Welcom to solsarin site ,Keep reading and find the answer about “is temperature intensive or extensive ”.
Stay with us.
Thank you for your support.


What is Intensive Property?
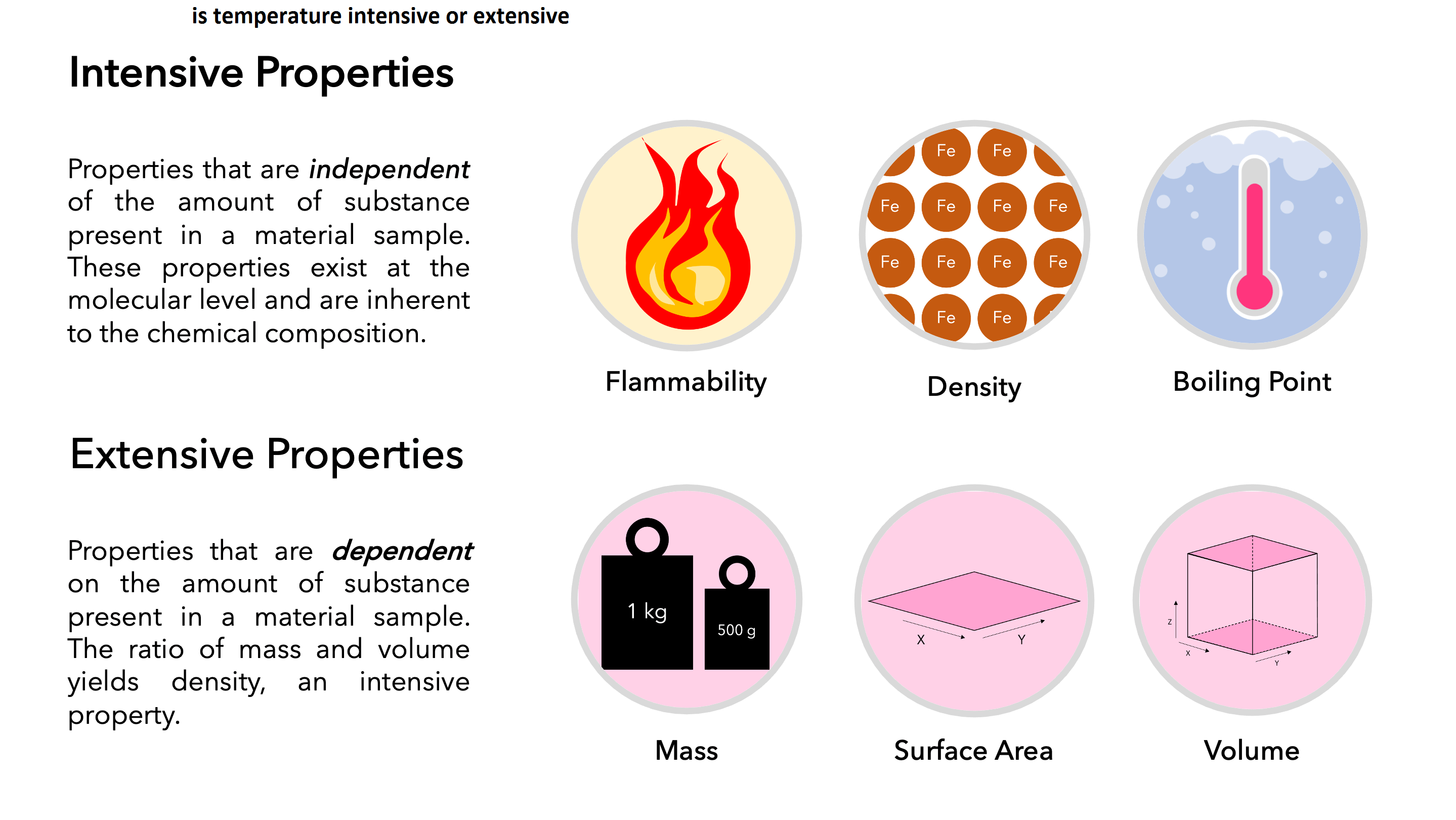
An intensive property is one that does not depend on the mass of the substance or system.
Temperature (T), pressure (P) and density (r) are examples of intensive properties.
Intensive Property Examples
The properties of matter that do not depend on the size or quantity of matter in any way are referred to as an intensive property of matter. Temperatures, density, color, melting and boiling point, etc., all are intensive property as they will not change with a change in size or quantity of matter. The density of 1 liter of water or 100 liters of water will remain the same as it is an intensive property.
What is Extensive property?
An extensive property of a system depends on the system size or the amount of matter in the system.
If the value of the property of a system is equal to the sum of the values for the parts of the system then such a property is called extensive property. Volume, energy, and mass are examples of extensive properties.
Extensive Property Examples
There are properties such as length, mass, volume, weight, etc. that depend on the quantity or size of the matter, these properties are called an extensive property of matter and their value changes if the size or quantity of matter changes. Suppose we have two boxes made up of the same material, one has a capacity of four litres while the other has a capacity of ten litres. The box with ten litres capacity will have more amount of matter as compared to that of a four-liter box.
Extensive Properties
Some properties of matter depend on the size of the sample, while some do not. An extensive property is a property that depends on the amount of matter in a sample. The mass of an object is a measure of the amount of matter that an object contains. A small sample of a certain type of matter will have a small mass, while a larger sample will have a greater mass. Another extensive property is volume. The volume of an object is a measure of the space that is occupied by that object.
The figure below illustrates the extensive property of volume. The pitcher and glass both contain milk. The pitcher holds approximately two quarts and the glass will hold about 8 ounces of milk. The same milk is in each container. The only difference is the amount of milk contained in the glass and in the pitcher.
Milk pitcher and glass.
Intensive Properties
The electrical conductivity of a substance is a property that depends only on the type of substance. Silver, gold, and copper are excellent conductors of electricity, while glass and plastic are poor conductors. A larger or smaller piece of glass will not change this property. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount. Other intensive properties include color, temperature, density, and solubility.
The copper wire shown in the picture below has a certain electrical conductivity. You could cut off the small end that sticks out, and it would have the same conductivity as the entire long roll of wire shown here. The conductivity is a property of the copper metal itself, not of the length of the wire.
Differences between Intensive and Extensive Properties
| Difference between Intensive and Extensive properties | |
|---|---|
| INTENSIVE | EXTENSIVE |
| Independent property | Dependent property |
| Size does not change | Size changes |
| It cannot be computed | It can be computed |
| Can be easily identified | Cannot be easily identified |
| Example: melting point, colour, ductility, conductivity, pressure, boiling point, lustre, freezing point, odour, density, etc | Example: length, mass, weight, volume |
Other Examples of Properties
Thermodynamics deals with the flow of heat energy. This flow of heat energy and its transformation into different forms is governed by the principles of thermodynamics. It depends on the matter and the factors that determine the state of a matter. The thermodynamic properties of a system depend on certain parameters. The parameters or variables are classified as state functions and path functions as defined below:
- State functions or state variables are those parameters that depend only on the current state of the system and not on the path that they have taken to reach this state.
For e.g: Temperature of the system.
- A path function is a parameter that depends on the path taken by the system to reach the current state.
For e.g: Work is done by frictional force.
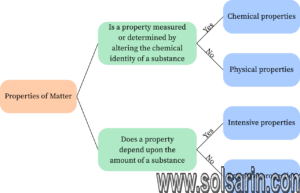
A state function depends only on the initial and final conditions while a path function depends on the path taken to reach the final condition from the initial condition. The thermodynamic properties of matter are also classified as intensive and extensive properties. This classification is based on the dependence of property on the size or quantity of matter under consideration. The intensive and extensive properties of matter are discussed below.
Recommended Videos
Frequently Asked Questions – FAQs
Which is the intensive property?
An intensive property, is a physical property of a system that does not depend on the system size or the amount of material in the system. According to the definitions, density, pressure and temperature are intensive properties and volume, internal energy are extensive properties.
What is the difference between intensive property and extensive property?
An extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount.
Is density a extensive property?
Density is an intensive property because there is a narrow range of densities across the samples. No matter what the initial mass was, densities were essentially the same. … Density is an intensive property of matter that illustrates how much mass a substance has in a given amount of volume.
Is work intensive or extensive?
Work is the product of Force (which is intensive) times distance (which is extensive). There are several distinct forms of ‘energy’ that are treated in thermodynamics. Pressure (an intensive property) times volume (an extensive property) is a form of energy.
Is weight an intensive or extensive property?
Extensive properties vary with the amount of the substance and include mass, weight, and volume. Intensive properties, in contrast, do not depend on the amount of the substance; they include color, melting point, boiling point, electrical conductivity, and physical state at a given temperature.
The intensive and extensive properties of matter help us in determining the thermodynamic state of a system; they provide us with the coordinates that are required to find the state of matter in thermodynamic terms. Call our mentors at BYJU’S for further support on intensive and extensive properties of matter.
is temperature intensive or extensive
is temperature intensive or extensive property
water is a liquid at room temperature intensive or extensive
Water can take many forms. At low temperatures (below 0oC0oC), water is a solid. When at “normal” temperatures (between 0oC0oC and 100oC100oC), it is a liquid. At temperatures above 100oC100oC, water is a gas (steam). The state of water depends on the temperature. Each state (solid, liquid, and gas) has its own unique set of physical properties.

Matter and its States
Matter typically exists in one of three states: solid, liquid, or gas. The state a given substance exhibits is also a physical property. Some substances exist as gases at room temperature (oxygen and carbon dioxide), while others, like water and mercury metal, exist as liquids. Most metals exist as solids at room temperature. All substances can exist in any of these three states.
Note
Technically speaking, a fourth state of matter called plasma exists. However, it does not naturally occur on earth, so we will omit it from our studies in this chapter.
Solid
Solids are defined by the following characteristics:
- Definite shape (rigid).
- Definite volume.
- Particles vibrate around fixed axes.
If we were to cool a sample of liquid mercury to its freezing point of −39oC−39oC, and had it contained under the right pressure conditions, we would notice all of the liquid particles would go into the solid state. Mercury can be solidified when its temperature is brought to its freezing point. However, when returned to room temperature conditions, mercury does not exist in solid state for long, and returns back to its more common liquid form.
Liquid
Liquids have the following characteristics:
- No definite shape (takes the shape of its container).
- Has definite volume.
- Particles are free to move over each other, but are still attracted to each other.
The aforementioned metal—Mercury—is an anomaly. It is the only metal we know of that is liquid at room temperature. Mercury also has an ability to stick to itself (surface tension), which is a property that all liquids exhibit. Mercury has a relatively high surface tension, and this makes it very unique. Pictured below is mercury in its common liquid form.
Mercury.
If we heat liquid mercury to its boiling point of 357oC357oC, and contain it under the right pressure conditions, we would notice all particles in the liquid state go into the gas state.
Summary
Three states of matter exist: solid, liquid, and gas. Solids have a definite shape and volume. Liquids have a definite volume, but take the shape of their container. Gases have no definite shape or volume.