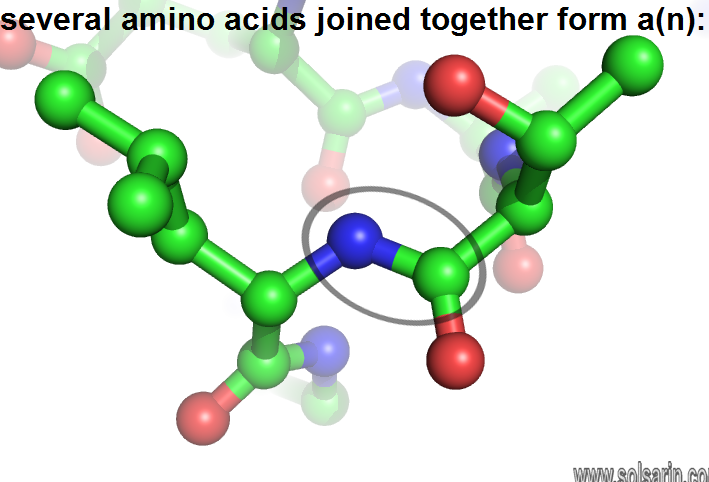
several amino acids joined together form a(n):
Hello dear friends, Thank you for choosing us. please join us on the solsarin site,In this post we will talk about “several amino acids joined together form a(n):”.
Stay with us.
Thank you fo r your choice.

Amino acids are the building blocks for proteins. All amino acids contain an amino or NH2 group and a carboxyl (acid) or COOH group. There are 20 different amino acids commonly found in proteins and often 300 or more amino acids per protein molecule. Each amino acid differs in terms of its “R” group.
The actual order of the amino acids in the protein is called its primary structure (Figure 19.1.419.1.4) and is determined by DNA. As will be seen later in this unit, DNA is divided into functional units called genes. A gene is a sequence of deoxyribonucleotide bases along one strand of DNA that codes for a functional product – a specific molecule of messenger RNA, transfer RNA, or ribosomal RNA. The product is usually messenger RNA (mRNA) and mRNA ultimately results in the synthesis of a polypeptide or a protein. Therefore, it is commonly said that the order of deoxyribonucleotide bases in a gene determines the amino acid sequence of a particular protein.
amino acid, any of a group of organic molecules that consist of a basic amino group (―NH2), an acidic carboxyl group (―COOH), and an organic R group (or side chain) that is unique to each amino acid. The term amino acid is short for α-amino [alpha-amino] carboxylic acid. Each molecule contains a central carbon (C) atom, called the α-carbon, to which both an amino and a carboxyl group are attached. The remaining two bonds of the α-carbon atom are generally satisfied by a hydrogen (H) atom and the R group. The formula of a general amino acid is:
The amino acids differ from each other in the particular chemical structure of the R group.
Figure 19.1.519.1.5
:
Secondary Structure of a Protein or Polypeptide. (left) The secondary structure of a protein or polypeptide is due to hydrogen bonds forming between an oxygen atom of one amino acid and a nitrogen atom of another. There are two possible types of secondary structure: an alpha helix and a beta sheet.
In the case of an alpha helix, the hydrogen bonding causes the polypeptide to twist into a helix. With a beta sheet the hydrogen bonding enables the polypeptide to fold back and forth upon itself like a pleated sheet. (right) The secondary structure of a protein or polypeptide is due to hydrogen bonds forming between an oxygen atom of one amino acid and a nitrogen atom of another. There are two possible types of secondary structure: an alpha helix and a beta sheet. In the case of an alpha helix, the hydrogen bonding causes the polypeptide to twist into a helix. With a beta sheet the hydrogen bonding enables the polypeptide to fold back and forth upon itself like a pleated sheet.
In globular proteins such as enzymes, the long chain of amino acids becomes folded into a three-dimensional functional shape or tertiary structure.
This is because certain amino acids with sulfhydryl or SH groups form disulfide (S-S) bonds with other amino acids in the same chain. Other interactions between R groups of amino acids such as hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions also contribute to the tertiary structure (Figure 19.1.619.1.6). In some proteins, such as antibody molecules and hemoglobin, several polypeptides may bond together to form a quaternary structure (Figure 19.1.719.1.7).
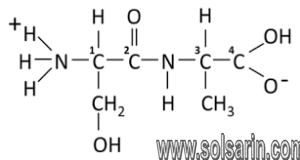

Figure 19.1.619.1.6
:
Tertiary Structure of a Protein or Polypeptide. In globular proteins such as enzymes, the long chain of amino acids becomes folded into a three-dimensional functional shape or tertiary structure. This is because certain amino acids with sulfhydryl or SH groups form disulfide (S-S) bonds with other amino acids in the same chain. Other interactions between R groups of amino acids such as hydrogen bonds, ionic bonds, covalent bonds, and hydrophobic interactions also contribute to the tertiary structure.
As will be seen later in this unit, during protein synthesis, the order of nucleotide bases along a gene gets transcribed into a complementary strand of mRNA which is then translated by tRNA into the correct order of amino acids for that polypeptide or protein.
Figure
19.1.719.1.7
:
Quaternary Structure of a Protein. The quaternary structure of a protein is due to several polypeptides joining together, as in the case of antibody molecules. Schematic diagram of the basic unit of immunoglobulin (antibody) Fab Fc heavy chain (consist of VH, CH1, hinge, CH2 and CH3 regions: from N-term) light chain (consist of VL and CL regions: from N-term) antigen binding site hinge regions (*) -S-S- mean disulfide bonds. (CC-SA-BY 3.0; Y_tambe).
Proteins Fold into a Conformation of Lowest Energy

As a result of all of these interactions, each type of protein has a particular three-dimensional structure, which is determined by the order of the amino acids in its chain. The final folded structure, or conformation, adopted by any polypeptide chain is generally the one in which the free energy is minimized.
Protein folding has been studied in a test tube by using highly purified proteins. A protein can be unfolded, or denatured, by treatment with certain solvents, which disrupt the noncovalent interactions holding the folded chain together. This treatment converts the protein into a flexible polypeptide chain that has lost its natural shape. When the denaturing solvent is removed, the protein often refolds spontaneously, or renatures, into its original conformation (Figure 3-8), indicating that all the information needed for specifying the three-dimensional shape of a protein is contained in its amino acid sequence.
Building blocks of proteins
Proteins are of primary importance to the continuing functioning of life on Earth. Proteins catalyze the vast majority of chemical reactions that occur in the cell. They provide many of the structural elements of a cell, and they help to bind cells together into tissues. Some proteins act as contractile elements to make movement possible. Others are responsible for the transport of vital materials from the outside of the cell (“extracellular”) to its inside (“intracellular”). Proteins, in the form of antibodies, protect animals from disease and, in the form of interferon, mount an intracellular attack against viruses that have eluded destruction by the antibodies and other immune system defenses. Many hormones are proteins. Last but certainly not least, proteins control the activity of genes (“gene expression”).
summary
- Amino acids are the building blocks for proteins. There are 20 different amino acids commonly found in proteins and often 300 or more amino acids per protein molecule.
- All amino acids contain an amino or NH2 group and a carboxyl (acid) or COOH group.
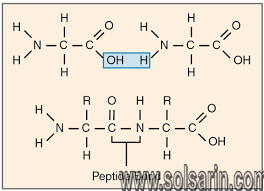
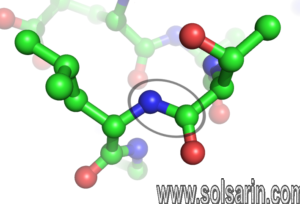
- To form polypeptides and proteins, amino acids are joined together by peptide bonds, in which the amino or NH2 of one amino acid bonds to the carboxyl (acid) or COOH group of another amino acid.
- A peptide is two or more amino acids joined together by peptide bonds; a polypeptide is a chain of many amino acids; and a protein contains one or more polypeptides. Therefore, proteins are long chains of amino acids held together by peptide bonds.
- The actual order of the amino acids in the protein is called its primary structure and is determined by DNA.
- The order of deoxyribonucleotide bases in a gene determines the amino acid sequence of a particular protein.