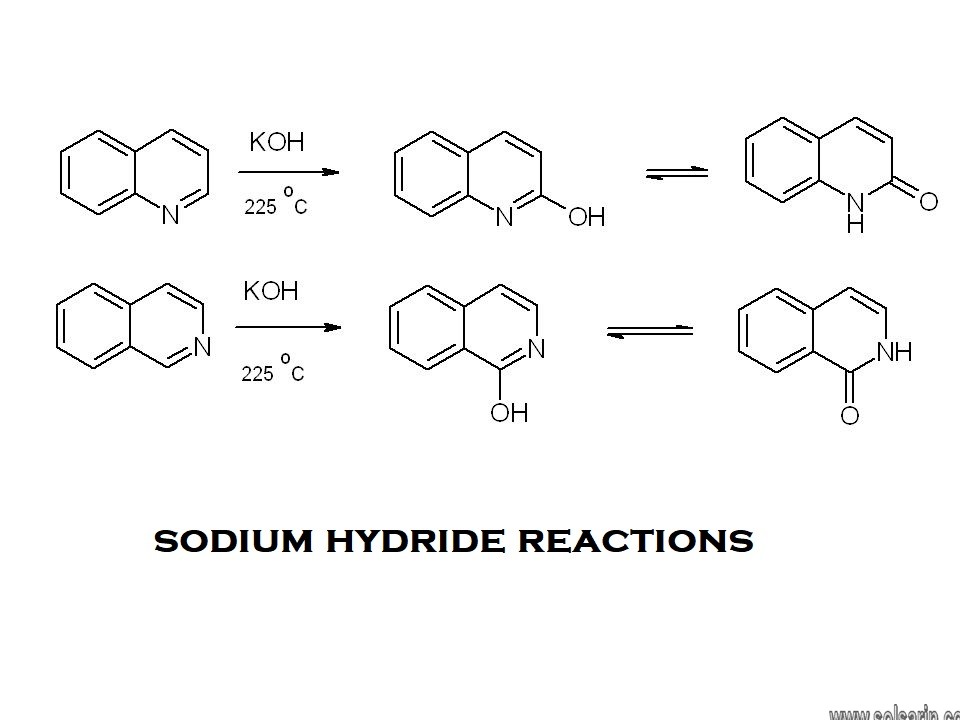
sodium hydride reactions
Hello. Welcome to solsarin. This post is about “sodium hydride reactions“.
Sodium hydride
How to Make It
Sodium hydride can be prepared by a direct reaction between hydrogen gas and sodium metal.
2 Na + H2 = 2NaH
Prominent Reactions
NaH reacts violently with water to produce sodium hydroxide and hydrogen gas that gets ignited by the heat of the reaction [5].
NaH + H2O = NaOH + H2
Alcohols react with sodium hydride to produce alkoxide ions. In the below reaction, ethanol reacts with NaH forming sodium ethoxide and hydrogen [10].
CH3CH2OH + NaH = CH3CH2O–Na+ + H2
Similarly, methanol reacts with sodium hydride producing the sodium salt of methanol and hydrogen gas.
CH3OH + NaH = CH3ONa + H2

Sodium Hydride Uses
- As a powerful reducing and deprotonating agent for many organic reactions [2].
- As a desiccant or drying agent for lab chemicals [2].
- Its application as a hydrogen storage agent in fuel cell vehicles is being explored due to its ability to release hydrogen [2].
- As a strong base, it finds application in organic and inorganic fine chemical preparation [6].
Is It Dangerous
NaH is a severe irritant to eyes, skin and respiratory system causing burns and is harmful if ingested. It is a highly inflammable and corrosive compound. Precaution must be taken against an explosion hazard if it comes in contact with water [2, 7]. When it is heated to the point of decomposition, it emits highly poisonous fumes of oxides of sodium [1]. After a reaction, unwanted sodium hydride should be destroyed by careful quenching or disposal [9].
Interesting Facts
- In spite of its high basicity, NaH is not nucleophilic [12].
Sodium Hydride (NaH) Price
It costs around $30-50 per kilogram. The price may vary with suppliers.
Abstract
The hazards associated with the thermal decomposition of chemically incompatible sodium hydride solvent matrices are known, with reports from the 1960s detailing the inherent instability of NaH/dimethyl sulfoxide, NaH/N,N-dimethylformamide, and NaH/N,N-dimethylacetamide mixtures. However, these hazards remain underappreciated and undercommunicated, likely as a consequence of the widespread use of these NaH/solvent matrices in synthetic chemistry.
We report herein detailed investigations into the thermal stability of these mixtures and studies of the formation of gaseous products from their thermal decomposition. We expect this contribution to promote awareness of these hazards within the wider scientific community, encourage scientists to identify and pursue safer alternatives, and most importantly, help to prevent incidents associated with these reactive mixtures.
Diphenylacetonitrile
During the course of our experiments on the α-methylation of diphenylacetonitrile (1) to prepare tertiary carbonitrile 2 a, we investigated its reaction with NaH (3 equiv) and MeI (1.2 equiv) in THF (85 °C in a sealed tube; Scheme 1). Although the desired tertiary nitrile 2 a was isolated in 74 % yield, we were surprised to observe the formation of 1,1-diphenylethane (3 a) in 25 % yield as a side product. Assuming that 3 a was formed by the decyanation of nitrile 2 a, we expected that this decyanation reaction could be generalized to a more versatile synthetic strategy. Therefore, we optimized the reaction conditions for the decyanation of nitrile 2 a by NaH (Table 1). We found that NaH alone was not sufficient to drive the decyanation (Table 1, entry 1).
Have you heard anything about “organisms that consume cellulose” ? Click on it.
Upon the methylation of 1 with NaH and MeI (Scheme 1), a stoichiometric amount of sodium iodide (NaI) is necessarily generated, and thus we speculated that the cooperation of NaH and NaI could be the key to the decyanation. Indeed, the treatment of 2 a with NaH (3 equiv) and NaI (2 equiv) in THF delivered 3 a in 96 % yield (Table 1, entry 2). Although KI was not optimally effective as an additive (Table 1, entry 3), LiI rendered the process very rapid to afford 3 a in 98 % yield within 3.5 h (entry 4).

Decyanation
Similarly, a reaction with MgI2 produced 3 a in 96 % yield, albeit at a slower reaction rate (Table 1, entry 5). Interestingly, LiBr or LiCl did not promote the decyanation effectively (Table 1, entries 6 and 7), thus indicating the important role of dissolved iodide ions in enabling this unprecedented decyanation by NaH. When the amount of LiI was decreased (Table 1, entries 8 and 9), we found that the use of even a catalytic amount of LiI (20 mol %) enabled full conversion of 2 a with a longer reaction time (48 h; entry 9).
When 1 equivalent of LiI was used as the promoter, the amount of NaH could be decreased to 1.5–2 equivalents (Table 1, entries 10 and 11). However, the reduction of 2 a with LiH in the presence of LiI did not proceed at all, thus indicating the specific reactivity of NaH for the present decyanation.
Introduction
DMSO
Do you want to know about “does glucose have protein” ? Click on it.
EXPERIMENTAL SECTION
Compound 3
1H NMR (500 MHz, CDCl3), δ 3.08 (s, 6H, 2×CH3), 5.13 (s, 4H, 2×CH2Ph). And 7.24 – 7.39 (m, 6H, Ar-H), 7.59 – 7.67 (m, 4H, Ar-H); 13C NMR (126 MHz, CDCl3), δ 47.8, 67.1, 127.3, 128.8, 130.3, 133.2; HRMS (FAB), calcd for C16H20N (M+), 226.1596, found 226.1599.
If you want to know about “how long does shingles pain last“, click on it.
Synthesis of Deuterated Compound 3
NaH (60% dispersion in oil, 20 mg, 0.5 mmol) added to a vigorously stirred solution of benzyl bromide. In DMF-d6 (0.7 mL) in ice-water bath and stirred for 16 h. The solution mixed with hexanes and the top hexanes layer removed. The DMF layer analyzed by NMR. The sample purified by recrystallization from chloroform.
Compound 3-D
1H NMR (500 MHz, DMF-d6) δ 5.24 (s, 4H), 7.43 – 7.52 (m, 6H), 7.73 – 7.80 (m, 4H); 13C NMR; 13C NMR (126 M Hz, DMF- d6) δ 46.5 (m), 65.8, 128.9, 130.3, 133.5, 139.5; HRMS (FAB), calcd for C16H14D6N (M+), 232.1972, found 232.1980.
Syntheses of Compounds 7, 8 and 9
Two methods used.

Method A
NaH (60% dispersion on oil, 0.2 g, 5 mmol) added to a vigorously stirred solution of benzyl bromide. In anhydrous acetonitrile in three portions over a period of 5 h in ice-water bath under a nitrogen atmosphere. The mixture stirred overnight, then it filtered through layers of silica gel and Celite and washed with acetonitrile. The combined filtrate mixed with hexanes and top hexanes layer removed. The acetonitrile layer evaporated down to an oil. Which distilled (130 °C/20 mmHg) to yield compound 8 as a major product (55%). The 1H NMR spectrum of this sample matched that of the authentic 3-phenylpropanenitrile purchased from Sigma-Aldrich.
Thank you for staying with this post “sodium hydride reactions” until the end.