table salt is formed when
Hello. Welcome to solsarin. This post is about “table salt is formed when“.
Salt
It is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantities in seawater. The open ocean has about 35 g (1.2 oz) of solids per liter of sea water, a salinity of 3.5%.
Salt is essential for life in general, and saltiness is one of the basic human tastes. Salt is one of the oldest and most ubiquitous food seasonings, and salting is an important method of food preservation.
Some of the earliest evidence of salt processing dates to around 6,000 BC, when people living in the area of present-day Romania boiled spring water to extract salts; a salt-works in China dates to approximately the same period. Salt was also prized by the ancient Hebrews, the Greeks, the Romans, the Byzantines, the Hittites, Egyptians, and the Indians.
Mediterranean Sea
Salt became an important article of trade and was transported by boat across the Mediterranean Sea, along specially built salt roads, and across the Sahara on camel caravans. The scarcity and universal need for salt have led nations to go to war over it and use it to raise tax revenues. Salt is used in religious ceremonies and has other cultural and traditional significance.

Salt is processed from salt mines, and by the evaporation of seawater (sea salt) and mineral-rich spring water in shallow pools. Its major industrial products are caustic soda and chlorine; salt is used in many industrial processes including the manufacture of polyvinyl chloride, plastics, paper pulp and many other products. Of the annual global production of around two hundred million tonnes of salt, about 6% is used for human consumption.
Other uses include water conditioning processes, de-icing highways, and agricultural use. Edible salt is sold in forms such as sea salt and table salt which usually contains an anti-caking agent and may be iodised to prevent iodine deficiency. As well as its use in cooking and at the table, salt is present in many processed foods.
less than 2,000 mg
Sodium is an essential nutrient for human health via its role as an electrolyte and osmotic solute.[1][2][3] Excessive salt consumption may increase the risk of cardiovascular diseases, such as hypertension, in children and adults. Such health effects of salt have long been studied. Accordingly, numerous world health associations and experts in developed countries recommend reducing consumption of popular salty foods. The World Health Organization recommends that adults consume less than 2,000 mg of sodium, equivalent to 5 grams of salt per day.[5][6]
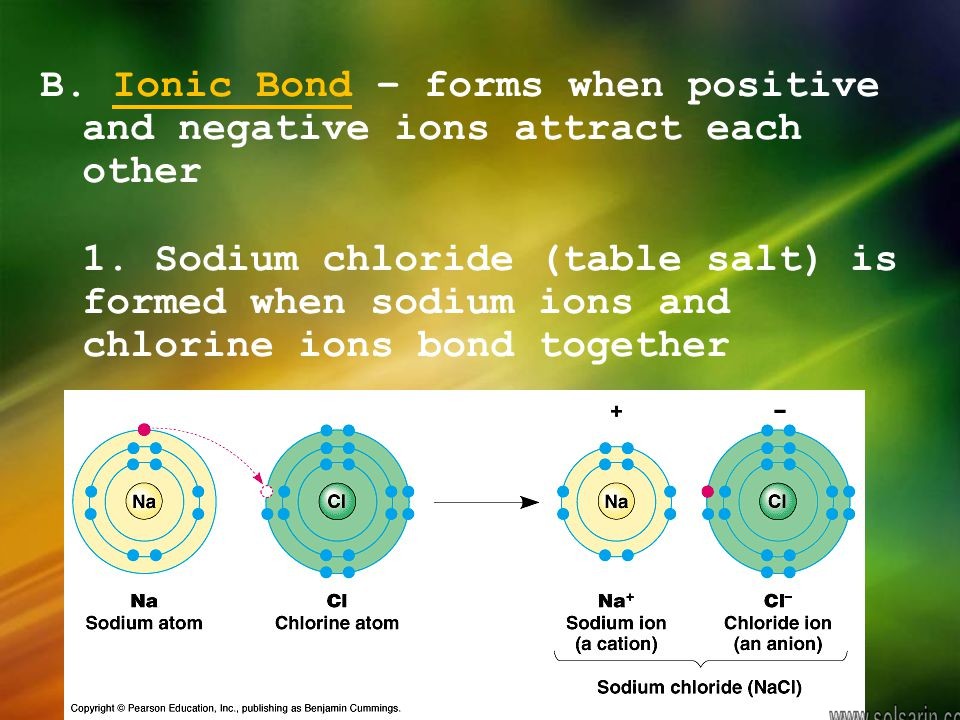
In terms of chemistry, a salt can be any compound formed by the reaction of an acid with a base. Energy, in the form of heat, is given off during this neutralization reaction so it is said to be exothermic. The most common salt, sodium chloride (NaCl), is a product of the reaction between hydrochloric acid (HCl) and the base sodium hydroxide (NaOH).
In this reaction, positively charged hydrogen ions (H+) from the acid are attracted to negatively charged hydroxyl ions (OH-) from the base. These ions combine and form water. After the water forms, the sodium and chlorine ions remain dissolved and the acid and base are said to be neutralized. Solid salt is formed when the water evaporates and the negatively charged chlorine ions combine with the positively charged sodium ions.
Crystals
Solid sodium chloride exists in the form of tiny, cube-shaped particles called crystals. These crystals are colorless, have a density of 2.165 g/cm3 and melt at 1,472°F (800.8°C). They also dissolve in water, separating into the component sodium and chlorine ions. This process known as ionization is important to many industrial chemical reactions.
Common salt (sodium chloride) is found throughout nature. It is dissolved in the oceans with an average concentration of 2.68%. On land, thick salt deposits, formed by the evaporation of prehistoric oceans, are widely distributed. These deposits are true sedimentary rocks and are referred to as rock salt or halite.
From the environment
People obtain salt from the environment in many different ways. Solid salt deposits are mined directly as rock salt and purified. Salt from sea water is isolated by solar evaporation. Underground salt deposits are solution-mined. This type of mining involves pumping water underground to dissolve the salt deposit, recovering the water with salt dissolved in it, and evaporating the water to isolate the salt.
Beyond being essential to the survival of most plants and animals, salt is also used extensively in many industries. In the food industry it is used to preserve meats and fish because it can slow down the growth of unhealthy microorganisms. It is also used to improve the flavor of many foods. In the cosmetic industry it is used to make soaps and shampoos. In other chemical industries it is the primary source of sodium and chlorine which are both raw materials used for various chemical reactions. Salt is used when manufacturing paper, rubber, and ceramics. And it is commonly used for de-icing roads during the winter.


How much is enough?
Surprisingly little is known about how much salt we need. U.S. residents consume, on average, about 3,400 milligrams of salt per day. For decades, the U.S. government and organizations, such as the American Heart Association, have recommended people consume less salt. Current dietary guidelines recommend no more than 2,300 mg of sodium—about a teaspoon of salt—per day for teens and adults. No more than 1,500 mg per day is recommended for groups at higher risk of heart disease, including African Americans and everyone over the age of 50.
1970s
The U.S. dietary guidelines were established in the 1970s when relatively little information was available about dietary salt and health. The guidelines were the best guess, given the information available at the time. However, the guidelines made little difference in people’s behavior. Between 1957 and 2003, U.S. residents consumed, on average, 3,400 mg of salt per day.
Also, over the years, salt consumption remained steady, even though manufacturers added more salt to commercial foods. This consistency in salt intake suggests that people may somehow automatically regulate the amount of salt they eat. If that is true, does it mean people instinctively choose the right amount of salt? Or perhaps people used less salt at the table in an attempt to follow the new guidelines but unknowingly consumed more salt in their prepared foods.
How much salt do you need?
If you are athletic and eat a low-salt diet, you may be getting too little salt. However, if you eat a lot of processed foods, you may be getting more salt than you need. As is probably clear to you by now, scientists and the medical establishment have not yet agreed on the optimal amount of salt people should get, but for most people, moderation is a good option.
You can estimate your daily intake of salt by paying attention to the nutrition labels on food. Some foods, including ready-to-eat foods such as items from the deli or bakery, are not required to have nutrition labels. If you eat a lot of these foods, or a lot of restaurant foods, it is harder to count your salt intake. However, you can find nutrition information for many restaurants, and deli and bakery items. Search for “nutrition information” and the name of the item. MyFitnessPal (www.myfitnesspal.com) also offers a free mobile app, and the online site lists nutrition information for thousands of products.
flavor
Blood tests can confirm your blood sodium level. If you discover that you eat too much salt, try to cut back on high-salt foods or add little or no salt to your food at the table. If food lacks flavor, you can try adding spices or herbs instead.
Salt is essential to the health of both people and animals. Table salt, used universally as a seasoning, is fine-grained and of high purity. To ensure that this hygroscopic (i.e., water-attracting) substance will remain free-flowing when exposed to the atmosphere, small quantities of sodium aluminosilicate, tricalcium phosphate, or magnesium silicate are added. Iodized salt—that is, salt to which small quantities of potassium iodide have been added—is widely used in areas where iodine is lacking from the diet, a deficiency that can cause swelling of the thyroid gland, commonly called goitre. Livestock also require salt; it is often made available in solid blocks.


Sodium Chloride Properties and Uses
Sodium chloride is vital for living organisms and important for industry. Most of the salinity of seawater is due to sodium chloride. The sodium and chloride ions are found in the blood, hemolymph, and extracellular fluids of multicellular organisms. Table salt is used to preserve food and enhance flavor. It’s also used to de-ice roads and walkways and as a chemical feedstock. Salt may be used as a cleaning agent. Fire extinguishers Met-L-X and Super D contain sodium chloride to extinguish metal fires.
Other Names: table salt, halite, sodium chloric
Chemical Formula: NaCl
Molar Mass: 58.44 grams per mole
Thank you for staying with this post “table salt is formed when” until the end.




