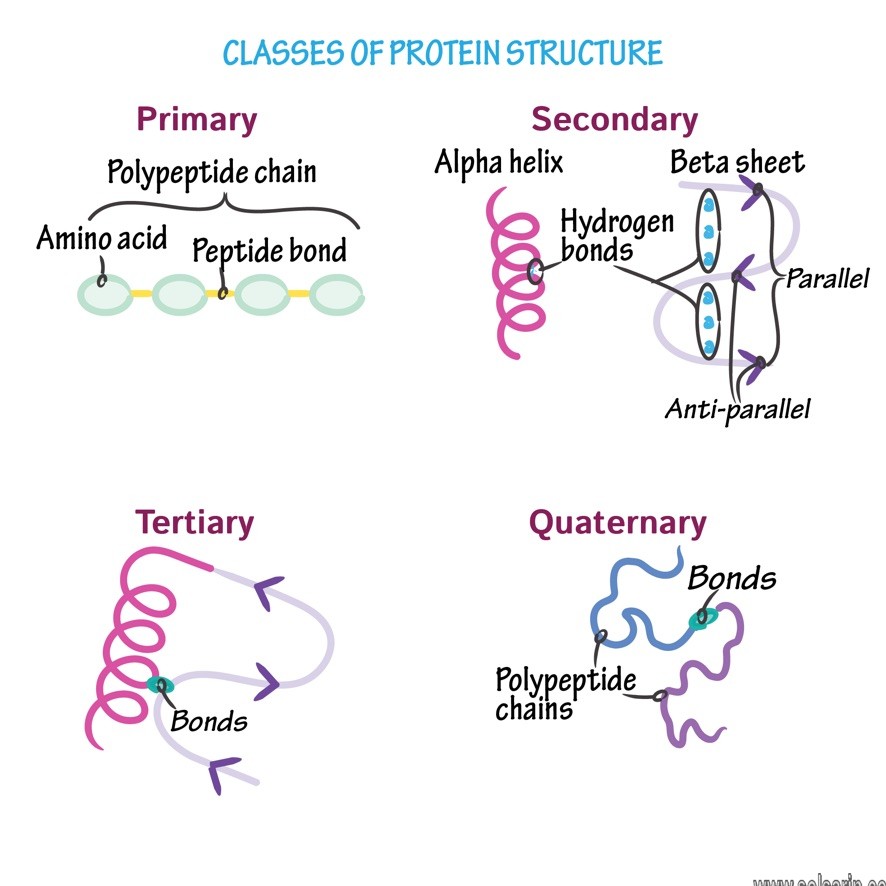
tertiary structure is not directly dependent on
Welcom to solsarin site ,Keep reading and find the answer about “tertiary structure is not directly dependent on”.
Stay with us.
Thank you for your support.
A peptide bond
A peptide bond, also referred to as an amide bond, is formed between the α-nitrogen atom of one amino acid and the carbonyl carbon of a second
So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than α-amine or α-carbonyl. In glutathione, for example, the γ-carboxyl group of glutamic acid is linked to the α-amino group of cysteine. During translation,
peptide bonds are formed from the amino (N) to the carboxyl (C) terminus by removal of water (also referred to as dehydration or condensation) and catalyzed by RNA (referred to as a ribozyme) that forms part of the ribosome.37 Peptides are also synthesized in vitro for therapeutic and experimental purposes.
Such chemical peptide synthesis proceeds from C to N terminus using N-protected amino acids and catalyzed by N,N’-dicylohexylcarbodiimide.38,39 In this scheme,
the nucleophilic amine group reacts with a carbodiimide : carbonyl intermediate, resulting in the formation of a new peptide bond and dicyclohexylurea. Dicyclohexylurea is insoluble in most solvents and can be easily removed from the maturing peptide. Cleavage of peptide bonds may be nonspecifically achieved by acid hydrolysis or accomplished specifically by a host of proteolytic enzymes with affinity for bonds between specific amino acid residues.
These protease systems are described later in this chapter.
Electron sharing in the amide bond (also known as the ω bond) is delocalized effectively preventing rotation about this bond.

This bond is fixed in one plane
This bond is fixed in one plane. Conformational flexibility of the peptide backbone results entirely from rotation about the axes of the two bonds to the α-carbon. Angles of rotation about these bonds are referred to as Ramachandran angles.40 The nitrogen to α-carbon bond angle is referred to as the Φ angle, and the α-carbon to carbonyl bond is referred to as the Ψ angle
Theoretically, free rotation about these bonds allows angles ranging from -180 to 180 degrees. In reality,
steric and energetic factors limit the possible combinations.
These bond angles play a key role in dictating the secondary structure of proteins. For example, values of Φ and Ψ in α-helices are approximately −60 and −45 degrees, respectively. Secondary structure is addressed more extensively later in this chapter.
What are examples peptide bonds?
For instance, a dipeptide is a peptide made up of two amino acids. A tripeptide is a peptide consisting of three amino acids. … The other peptide bond is the isopeptide bond, i.e. a peptide bond formed between the carboxyl group and an amino group of joining amino acids at position other than the alpha.
Structure of an Amino Acid
Amino acids are the monomers that make up proteins. Each amino acid has the same fundamental structure, which consists of a central carbon atom,
also known as the alpha (α) carbon, bonded to an amino group (NH2), a carboxyl group (COOH),
and to a hydrogen atom. In the aqueous environment of the cell,
the both the amino group and the carboxyl group are ionized under physiological conditions, and so have the structures -NH3+ and -COO–, respectively.
Every amino acid also has another atom or group of atoms bonded to the central atom known as the R group.
This R group, or side chain, gives each amino acid proteins specific characteristics, including size, polarity, and pH.

Types of Amino Acids
The name “amino acid” is derived from the amino group and carboxyl-acid-group in their basic structure. There are 21 amino acids present in proteins, each with a specific R group or side chain.
Ten of these are considered essential amino acids in humans because the human body cannot produce them and they must be obtained from the diet.
All organisms have different essential amino acids based on their physiology.

Characteristics of Amino Acids
Which categories of amino acid would you expect to find on the surface of a soluble protein, and which would you expect to find in the interior?
What distribution of amino acids would you expect to find in a protein embedded in a lipid bilayer?
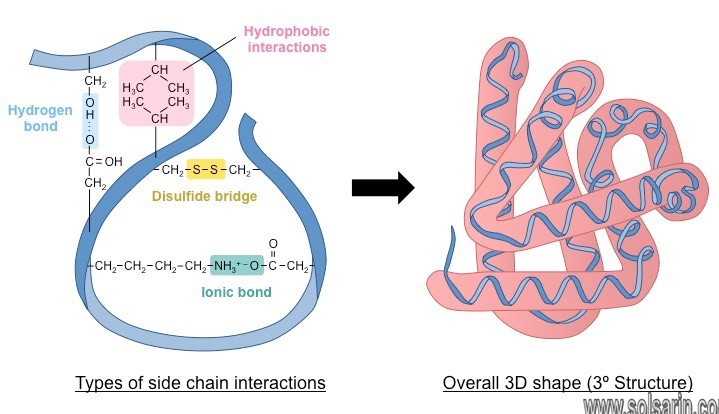
The chemical composition of the side chain determines the characteristics of the amino acid.
Amino acids such as valine, methionine, and alanine are nonpolar (hydrophobic),
while amino acids such as serine,
threonine, and cysteine are polar (hydrophilic).
The side chains of lysine and arginine are positively charged so these amino acids are also known as basic (high pH) amino acids. Proline is an exception to the standard structure of an amino acid because its R group is linked to the amino group, forming a ring-like structure.
Amino acids are represented by a single upper case letter or a three-letter abbreviation. For example, valine is known by the letter V or the three-letter symbol val.
Polypeptide Chains
The resulting chain of amino acids is called a polypeptide chain. Each polypeptide has a free amino group at one end. This end is called the N terminal, or the amino terminal, and the other end has a free carboxyl group, also known as the C or carboxyl terminal. When reading or reporting the amino acid sequence of a protein or polypeptide,
the convention is to use the N-to-C direction. That is, the first amino acid in the sequence is assumed to the be one at the N terminal and the last amino acid is assumed to be the one at the C terminal.
Although the terms polypeptide and protein are sometimes used interchangeably,
a polypeptide is technically any polymer of amino acids, whereas the term protein is used for a polypeptide or polypeptides that have folded properly, combined with any additional components needed for proper functioning, and is now functional.

Peptide Bonding between Amino Acids
The peptide bond is an amide bond which links amino acids together to form proteins
What is the difference between a hydrogen bond and a peptide bond?
A peptide bond, or a covalent chemical bond between two molecules, binds the two amino acids together. … Hydrogen bonding, not a covalent bond to a hydrogen atom, is a special form of dipole-dipole attraction between molecules.
How is a peptide bond formed?
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O).
This is a dehydration synthesis reaction (also known as a condensation reaction),
and usually occurs between amino acids.
The resulting CO-NH bond is called a peptide bond,
and the resulting molecule is an amide.
The four-atom functional group -C(=O)NH- is called an amide group or (in the context of proteins) a peptide group.
Polypeptides and proteins are chains of amino acids held together by peptide bonds, as is the backbone of PNA.
Which is stronger peptide bond or hydrogen bond?
The reason for this is that the atoms in peptide bonds carry more charge than the hydrogen and oxygen atoms in water molecules, and interactions that involve more charge are stronger.
Thus, a hydrogen bond between two peptide bonds is stronger than a hydrogen bond between a peptide bond and water.
Are peptide bonds polar or nonpolar?
Polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. Hence the peptide bond is a nonpolar covalent bond because it holds together two amino acids.
Hence the peptide bond is nonpolar.
What molecule has peptide bonds?
A protein molecule is a made up of large number of amino acids, arranged in a chain, bound by peptide bonds.
Each amino acid has an amino group ( NH2 ) and a carboxyl group ( COOH ) .
Why are peptide bonds stable?
The stability of the peptide bond is due to the resonance of amides. With resonance, the nitrogen is able to donate its lone pair of electrons to the carbonyl carbon and push electrons from the carbonyl double bond towards the oxygen, forming the oxygen anion.
What is the strongest bond in proteins?
Covalent bonds are the strongest chemical bonds contributing to protein structure. Covalent bonds arise when two atoms share electrons.
Why peptide bonds are polar?
A polypeptide chain contains a sequence of amino acids joined by peptide bonds,
and each amino acid unit in a polypeptide is called a residue.
With an alpha-amino group at one end and an alpha-carboxyl group at the other, a polypeptide chain has polarity because its ends are distinct.
Are peptide bonds hydrophilic?
These R-groups are neither strongly hydrophilic nor hydrophobic. Atoms in long molecules, such as polypeptides, are not rigidly fixed in space or position.
The covalent bonds that hold them together allow the atoms to rotate and take up a three-dimensional position in the molecule where they are the most stable.



