the disaccharides include:
Hello. Welcome to solsarin. This post is about “the disaccharides include:“.
Disaccharide
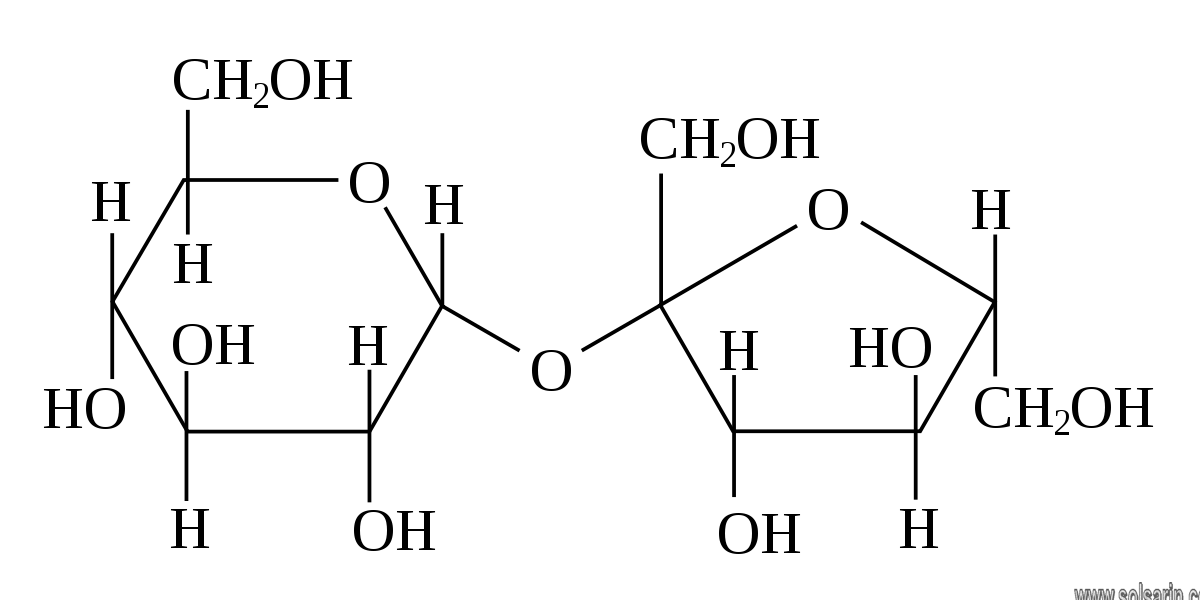
A disaccharide (also called a double sugar or biose) is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose.
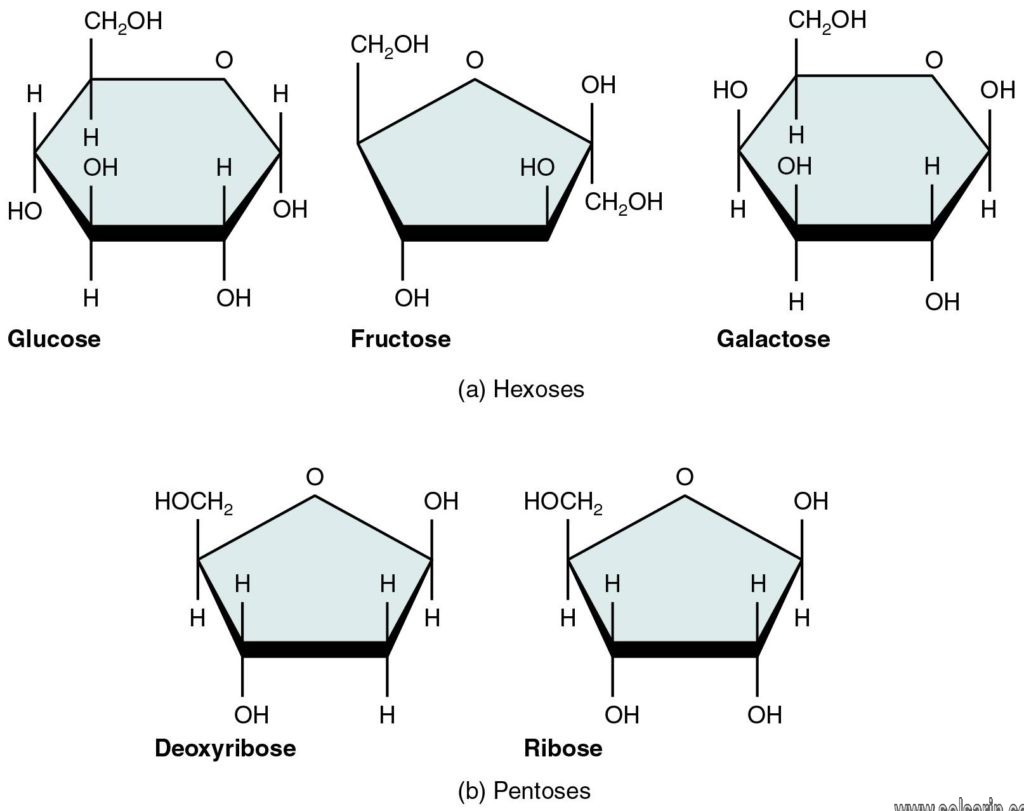
Disaccharides are one of the four chemical groupings of carbohydrates (monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule.
Sucrase, lactase, and maltase
The joining of monosaccharides into a double sugar happens by a condensation reaction, which involves the elimination of a water molecule from the functional groups only. Breaking apart a double sugar into its two monosaccharides is accomplished by hydrolysis with the help of a type of enzyme called a disaccharidase. As building the larger sugar ejects a water molecule, breaking it down consumes a water molecule. These reactions are vital in metabolism. Each disaccharide is broken down with the help of a corresponding disaccharidase (sucrase, lactase, and maltase).

Classification
There are two functionally different classes of disaccharides:
- Reducing disaccharides, in which one monosaccharide, the reducing sugar of the pair, still has a free hemiacetal unit that can perform as a reducing aldehyde group; lactose, maltose and cellobiose are examples of reducing disaccharides, each with one hemiacetal unit, the other occupied by the glycosidic bond, which prevents it from acting as a reducing agent. They can easily be detected by the Woehlk test or Fearon’s test on methylamine.
- Non-reducing disaccharides, in which the component monosaccharides bond through an acetal linkage between their anomeric centers. This results in neither monosaccharide being left with a hemiacetal unit that is free to act as a reducing agent. Sucrose and trehalose are examples of non-reducing disaccharides because their glycosidic bond is between their respective hemiacetal carbon atoms. The reduced chemical reactivity of the non-reducing sugars in comparison to reducing sugars, may be an advantage where stability in storage is important.
α-,β-linkage
Sucrose, which is formed following photosynthesis in green plants, consists of one molecule of glucose and one of fructose bonded via an α-,β-linkage. Lactose (milk sugar), found in the milk of all mammals, consists of glucose and galactose connected by a β-linkage. Maltose, a product of the breakdown of starches during digestion, consists of two molecules of glucose connected via an α-linkage. Another important disaccharide, trehalose, which is found in single-celled organisms and in many insects, also consists of two molecules of glucose and an α-linkage, but the linkage is distinct from the one found in maltose.
Here is a list of some disaccharides, including the monosaccharides they are made from and foods containing them. Sucrose, maltose, and lactose are the most familiar disaccharides, but there are others.
Sucrose (saccharose)
glucose + fructose
Sucrose is table sugar. It is purified from sugar cane or sugar beets.
Maltose
glucose + glucose
Maltose is a sugar found in some cereals and candies. It is a product of starch digestions and may be purified from barley and other grains.
Lactose
galactose + glucose
Lactose is a disaccharide found in milk. It has the formula C12H22O11 and is an isomer of sucros.
Lactulose
galactose + fructose
Lactulose is a synthetic (man-made) sugar that is not absorbed by the body but is broken down in the colon into products that absorb water into the colon, thus softening stools. Its primary use is to treat constipation.1 It is also used to reduce blood ammonia levels in persons with liver disease since lactulose absorbs ammonia into the colon (removing it from the body).2
Trehalose
glucose + glucose
Trehalose is also known as tremalose or mycose. It is a natural alpha-linked disaccharide with extremely high water retention properties. In nature, it helps plants and animals reduce long periods without water.

Cellobiose
glucose + glucose
Cellobiose is a hydrolysis product of cellulose or cellulose-rich materials, such as paper or cotton. It is formed by linking two beta-glucose molecules by a β(1→4) bond.
Bonds and Properties
Note multiple disaccharides are possible when monosaccharides bond to each other, since a glycosidic bond can form between any hydroxyl group on the component sugars. For example, two glucose molecules can join to form maltose, trehalose, or cellobiose. Even though these disaccharides are made from the same component sugars, they are distinct molecules with different chemical and physical properties from each other.
Uses of Disaccharides
Disaccharides are used as energy carriers and to efficiently transport monosaccharides. Specific examples of uses include:
- In the human body and in other animals, sucrose is digested and broken into its component simple sugars for quick energy. Excess sucrose can be converted from a carbohydrate into a lipid for storage as fat. Sucrose has a sweet flavor.
- Lactose (milk sugar) is found in human breast milk, where it serves as a chemical energy source for infants. Lactose, like sucrose, has a sweet flavor. As humans age, lactose becomes less-tolerated. This is because lactose digestion requires the enyzme lactase. People who are lactose intolerant can take a lactase supplement to reduce bloating, cramping, nausea, and diarrhea.3
- Plants use disaccharides to transport fructose, glucose, and galactose from one cell to another.
- Maltose, unlike some other disaccharides, does not serve a specific purpose in the human body. The sugar alcohol form of maltose is maltitol, which is used in sugar-free foods. Of course, maltose is a sugar, but it is incompletely digested and absorbed by the body (50–60%).
What happens to disaccharides during digestion?
Disaccharides are a form of carbohydrate that can be found in a wide variety of the foods we eat, such as table sugar and beetroot. Disaccharides are an energy source used by the human body, but are also used by plants for a variety of different uses (including transporting nutrients around the plant).
A disaccharide can also be classified as a double sugar as it is formed by two monosaccharides (also called single sugars) to create a disaccharide. Three of the most common disaccharides we consume are sucrose, maltose and lactose. They are found in many everyday whole foods and packaged foods we purchase from supermarkets or stores. Other less common disaccharides include lactulose, trehalose, and cellobiose, which can be found in foods like raw milk, mushrooms, some seaweeds and honey.
Chocolate or lollies
When we consume disaccharides our bodies break them down into single sugars. These sugars are glucose, fructose and galactose, and they are used as energy for our body. Lactose, for example, can be found in breast milk and is used as an energy source by infants. Likewise, maltose is a sweetener that’s regularly used in confectionery like chocolate or lollies.
What happens to disaccharides during the digestion process?
As disaccharides travel through the body they are broken down into simple sugars, or monosaccharides, by a process called hydrolysis. This process is facilitated by enzymes called maltases, sucrases, and lactases.
These different enzymes help to break down different types of sugars in the body. For example, maltase breaks down maltose into glucose, sucrases help break down sucrose into glucose and fructose, and lactases break down lactose into glucose and galactose.
What part of the body are disaccharides processed?
When we consume carbohydrates, our body will break these down into single sugars (monosaccharides) for digestion, absorption and transportation.
If you want to know about “how do fungi obtain nutrients“, click on it.
Carbohydrate digestion starts almost immediately in the mouth with salivary amylase (an enzyme) being released during the process of chewing. Minimal carbohydrate digestion happens in the stomach due to the fact that salivary amylase is sensitive to pH and thus inhibited in the acidic environment of the human stomach. Once food moves from the stomach to the small intestine, the enzymes listed above begin to break down disaccharides. This occurs in the microvillus membrane (brush border) found in the inner wall of the small intestine.
GLUT5
The body then starts to absorb and transport different types of sugars around the body for use as energy. This happens via a variety of transporters located in the wall of the small intestine. Glucose and galactose are transported by the SGLT-1 transporter (sodium-glucose co-transporter) and fructose is absorbed by the GLUT5 transporter.
Digestion and absorption are coupled with enzymes closely located to the appropriate transporters to enable the body to utilise single sugars (monosaccharides) as an energy source.

Formation and Breakdown of Disaccharides
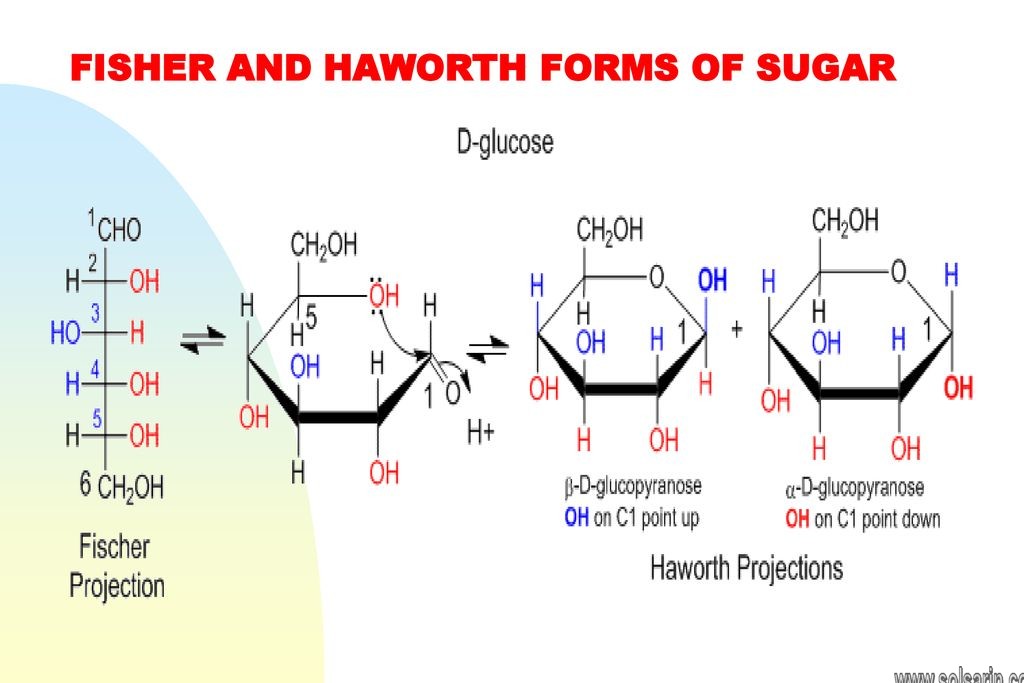
When disaccharides are formed from monosaccharides, an -OH (hydroxyl) group is removed from one molecule and an H (hydrogen) is removed from the other. Glycosidic bonds are formed to join the molecules; these are covalent bonds between a carbohydrate molecule and another group (which does not necessarily need to be another carbohydrate). The H and -OH that were removed from the two monosaccharides join together to form a water molecule, H2O. For this reason, the process of forming a disaccharide from two monosaccharides is called a dehydration reaction or condensation reaction.
Thank you for staying with this post “the disaccharides include:” until the end.




