the smallest particle of an element
Hello. Welcome to solsarin. Tonight’s post is about “the smallest particle of an element“.
An element is a substance completely made up of one atom. Thus, the periodic table of elements is effectively a list of all known types of atoms. However, the atom itself is not the smallest known particle, but instead each atom is made up of three individual parts: electrons, protons and neutrons. Furthermore, protons and neutrons themselves are made up of even smaller parts called quarks.
Electrons
They are fundamental particles, which means no particle is known to make up an electron. Electrons are what give an atom of an element its charge; you can change the number of electrons to make it a positively- or negatively-charged version of the same atom. A neutrally-charged atom will have the same amount of electrons as protons. They exists in orbitals, which surround the nucleus of the atoms, and it is in these orbitals that electrons can bond with other atoms to form compounds.


Protons
Protons are the defining characteristic of an element’s atom; the number of protons is what gives the atom its mass (electrons have a negligible amount of mass in comparison to protons). Thus, elements are classified by the number of protons its atoms have and organized in such a way on the periodic table (e.g., a hydrogen atom has one proton, a carbon atom has six, etc.). Protons are found in the nucleus of the atom.
Neutrons
Neutrons are about as massive as protons, and are found in the nucleus of the atom alongside protons. While protons have a positive charge and electrons have a negative charge, neutrons have no charge. Much how changing the number of electrons does not change the element itself, changing the amount of neutrons keeps relatively the same type of element, but creates an isotope. Isotopes can be unstable, and when they decay, they release energy in the form of radiation.
Quarks
Electrons are fundamental particles; however, protons and neutrons are made up of a different set of fundamental particles known as quarks. Discovered in 1961, quarks are the smallest known particles in physics, and there are six types (up, down, charm, strange, bottom and top). Three quarks combine together to form baryons, which include protons and neutrons. A quark can also combine in a pair with an antiquark to form a meson, but this type of matter is extremely unstable and lasts for only a fraction of a millisecond.
Atom
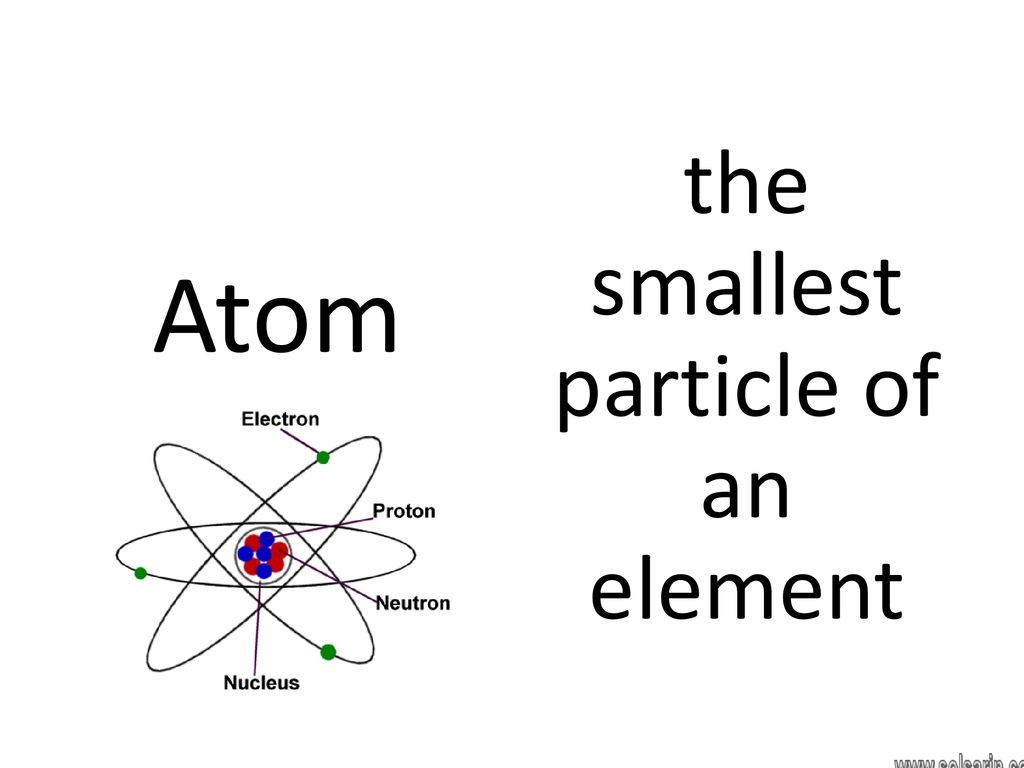
An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.
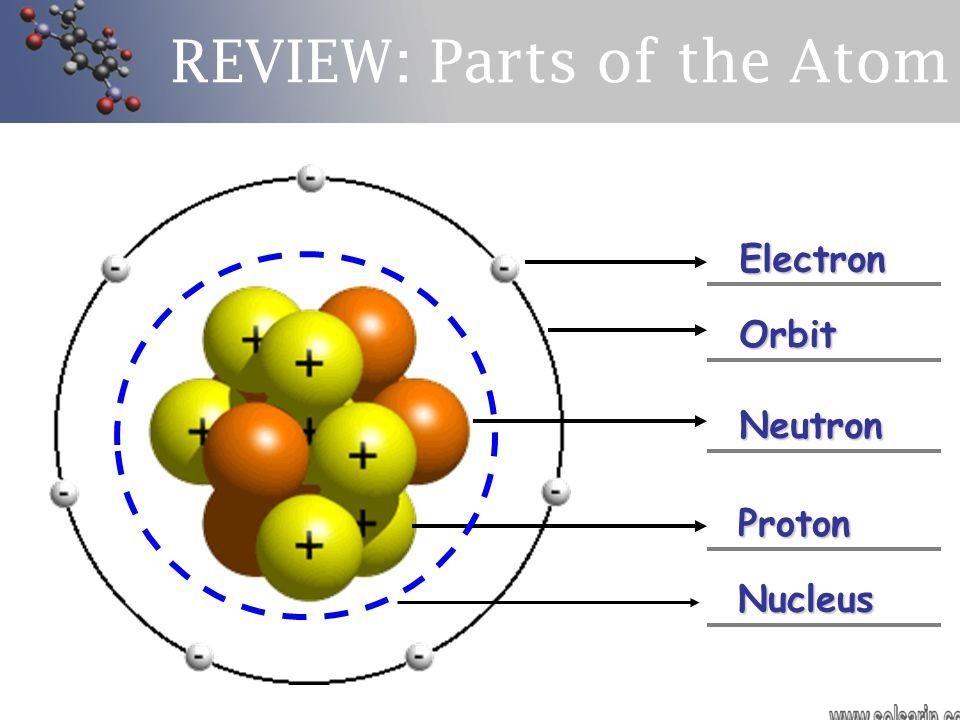
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. More than 99.94% of an atom’s mass is in the nucleus.
The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions.
Electromagnetic
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus splits and leaves behind different elements. This is a form of nuclear decay.
The number of protons in the nucleus is the atomic number and it defines to which chemical element the atom belongs. For example, any atom that contains 29 protons is copper. The number of neutrons defines the isotope of the element. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature. Chemistry is the discipline that studies these changes.

Lincoln
Physicists use electron volts (eV) to measure the mass of subatomic particles, Lincoln said. Technically, the unit is eV/c^2, in which c is the speed of light. One electron volt is equivalent to about 1.6×10^-19 joules. To simplify things, physicists use a set of units whereby the speed of light is 1. To figure out the mass of a subatomic particle, then, you’d use Albert Einstein’s famous equation E=mc^2 to get the mass (m) in kilograms.
An electron weighs 511,000 electron volts, which is equivalent to 9.11 x 10^-31 kilograms, according to Lincoln. For comparison, a typical proton in the nucleus of a typical atom weighs 938 million electron volts, or 1.67 × 10^-27 kg, he said.
172.5 billion
Conversely, the largest (in terms of mass) fundamental particle we know of is a particle called a top quark, measuring a whopping 172.5 billion electron volts, according to Lincoln. Quarks are another fundamental particle that, as far as we know, cannot be broken down into more parts. Scientists have found six types of quarks: up, down, strange, charm, bottom and top. Up and down quarks make up protons and neutrons, and they weigh 3 million and 5 million electron volts, respectively. In comparison, the top quark weighs 57,500 times more than the up quark.
The question of physical size is harder to answer. We know the physical size of some particles, but not the smallest ones. Some “tiny” particles that people hear about in daily life, such as virus particles, are actually quite large.
250 to 400
Lincoln offered this sense of scale: A typical virus particle is about 250 to 400 nanometers long (a nanometer is a billionth of a meter, or 10^-9 m), and the typical atomic nucleus measures about 10^-14 m (0.00000000000001 m). That means an atomic nucleus is as small to a virus as a virus is to us.
Currently, the smallest physical size scientists can measure with a particle accelerator is 2,000 times smaller than a proton, or 5 x 10^-20 m. So far, scientists have been able to determine that quarks are smaller than that, but not by how much.
What is the smallest particle of an element that can exist called?
Atom is the smallest particle of every element. They are called the building block in chemistry. Atoms are extremely small in size. They cannot be seen by naked eyes. Atoms are present deep inside every element. It contains protons, neutrons and electrons.
The smallest particle of an element that can exist is called an atom. It is the smallest unit of every element. It is also called the basic building block of chemistry. The idea of the atom was first introduced by Democritus, almost 25002500 years ago.
All matter is made up of atoms.
Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. These are extremely small particles typically around 100100 picometers approx. All atoms are roughly the same size. Every atom is composed of a nucleus surrounded by a cloud of negatively charged electrons. The nucleus is made of one or more positively charged protons and several neutrons. Most spaces in the atom are empty spaces and the rest space is occupied by protons, neutrons, and electrons. Despite the small size of the nucleus, virtually all the mass of the atom is concentrated there. The atomic number is the number of protons an atom has. It is characteristic and unique for each element.

Different properties
Whenever there is an addition of an atom then there is the formation of a new element, and whenever there is the addition of a neutron, there is the formation of an isotope or a heavier version of that particular atom. Atoms have different properties based on the arrangement and number of their basic particles.
Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. They are attracted to any positive charge by their electric force; in an atom, electric forces bind the electrons to the nucleus.
Rotate one core
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties.
In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells.
This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. For additional information pertaining to nuclear structure and elementary particles, see subatomic particles.

I hope you enjoyed this post “the smallest particle of an element”.