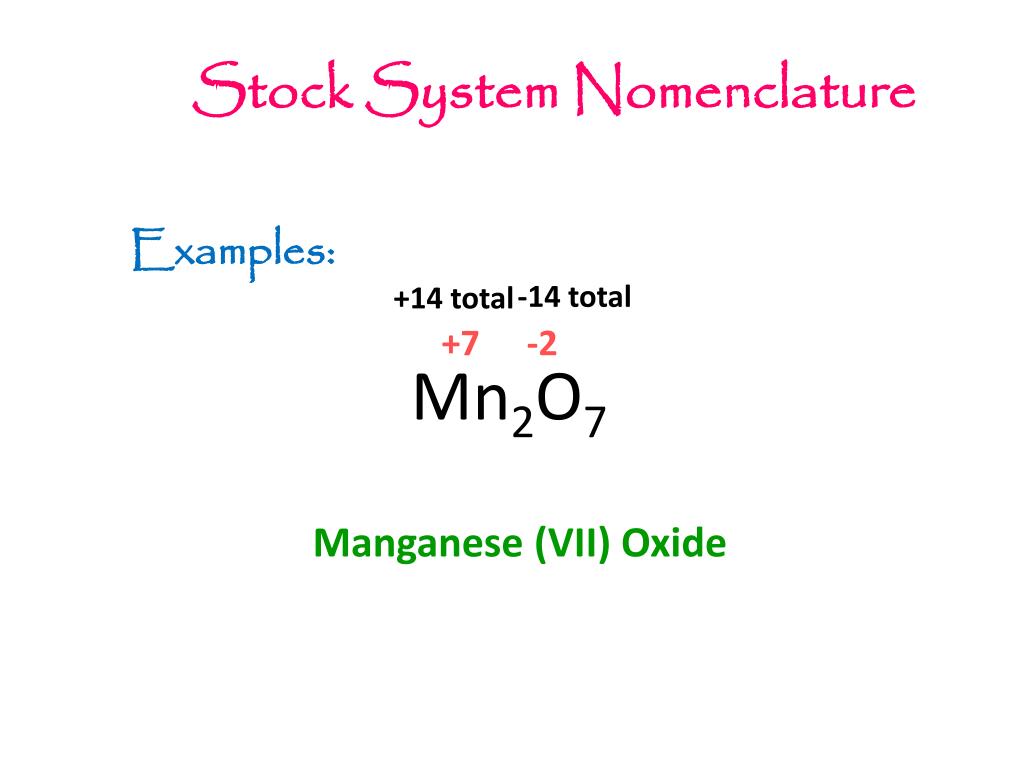
the stock system name for mn2o7 is
Welcome to solsarin, Keep reading and find the answer about “the stock system name for mn2o7 is”.

In the description of many technological items, it’s not enough to simply state a brand or model. Details are discussed such as how much horsepower is “under the hood” for a car, or how fast the chip is for a computer. Even a simple device like an mp3 player has more than one size. One can purchase an 8MB player, or a 16MB player. Designation of the item is often incomplete without other information as to its capabilities.
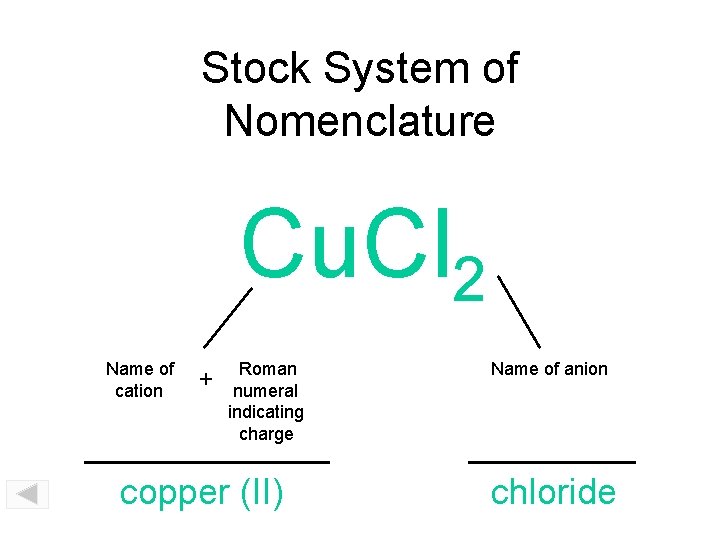
Transition metals have more than one possibility for ion formation. In order to name these compounds correctly, it’s important to indicate which ion is involved in any given compound.
Naming Compounds Using the Stock System
Naming compounds that involve transition metal cations necessitates the use of the Stock system. Consider the binary ionic compound \(\ce{FeCl_3}\). To simply name this compound “iron chloride” would be incomplete, because iron is capable of forming two ions with different charges. The name of any iron-containing compound must reflect which iron ion is in the compound. In this case, the subscript in the formula indicates that there are three chloride ions, each with a \(1-\) charge. Therefore, the charge of the single iron ion must be \(3+\). The correct name of \(\ce{FeCl_3}\) is iron (III) chloride, with the cation charge written as the Roman numeral. Here are several other examples:
| Formula | Name |
|---|---|
| \(\ce{Cu_2O}\) | copper (I) oxide |
| \(\ce{CuO}\) | copper (II) oxide |
| \(\ce{SnO_2}\) | tin (IV) oxide |
The first two examples are both oxides of copper (shown in the figure below). The ratio of copper ions to oxide ions determines the name. Since the oxide ion is \(\ce{O^{2-}}\), the charges of the copper ion must be \(1+\) in the first formula and \(2+\) in the second formula. In the third formula, there is one tin ion for every two oxide ions. This means that the tin must carry a \(4+\) charge, making the name tin (IV) oxide.
Manganese heptoxide
Manganese(VII) oxide (manganese heptoxide) is an inorganic compound with the formula Mn2O7. This volatile liquid is highly reactive. It is a dangerous oxidizer and was first described in 1860. It is the acid anhydride of permanganic acid.
Properties
The crystalline form of this chemical compound is dark green. The liquid is green by reflected light and red by transmitted light. It is soluble in carbon tetrachloride and decomposes when in contact with water.
Structure
Its solubility properties indicate a nonpolar molecular species, which is confirmed by its structure. The molecules consist of a pair of tetrahedra that share a common vertex. The vertices are occupied by oxygen atoms and at the centers of the tetrahedra are the Mn(VII) centers. The connectivity is indicated by the formula O3Mn−O−MnO3. The terminal Mn−O distances are 1.585 Å and the bridging oxygen is 1.77 Å distant from the two Mn atoms. The Mn−O−Mn angle is 120.7°.
Pyrosulfate, pyrophosphate, and dichromate adopt structures similar to that of Mn2O7. Probably the most similar main group species is Cl2O7. Focusing on comparisons within the transition metal series, Tc2O7 and Mn2O7 are structurally similar but the Tc−O−Tc angle is 180°. Solid Re2O7 is not molecular but consists of crosslinked Re centers with both tetrahedral and octahedral sites; in the vapor phase, it is molecular with a similar structure to Tc2O7.
Read More Posts:
Synthesis and reactions
Mn2O7 arises as a dark green oil by the addition of concentrated H2SO4 to KMnO4. The reaction initially produces permanganic acid, HMnO4 (structurally, HOMnO3), which is dehydrated by cold sulfuric acid to form its anhydride, Mn2O7.
- 2 KMnO4 + 2 H2SO4 → Mn2O7 + H2O + 2 KHSO4
Mn2O7 can react further with sulfuric acid to give the remarkable mangal(VII) cation MnO+
3, which is isoelectronic with CrO3
- Mn2O7 + 2 H2SO4 → 2 [MnO
3]+
[HSO
4]−
+ H2O
Mn2O7 decomposes near room temperature, explosively so above 55 °C. The explosion can be initiated by striking the sample or by its exposure to oxidizable organic compounds. The products are MnO2 and O2. Ozone is also produced, giving a strong smell to the substance. The ozone can spontaneously ignite a piece of paper impregnated with an alcohol solution.
Manganese heptoxide reacts with hydrogen peroxide in presence of sulfuric acid, liberating oxygen and ozone:
Naming Compounds
• Goal: Names from formulas; Formulas from names• Know Tables 2.3 – 2.6 well – Do Homework!• Learn elemental symbols and names for elements 1-36 and other common elements
1. Binary (contains two elements) ionic compounds- contain typically a metal cation and a non-metal anion
Two Types
Type I: contains simple monoatomic cation and anion
Rules:– cation is named first– for simple, monoatomic cations, use element name(ex. K+ = potassium)Note: alkali metals (Group IA) form 1+ cationsalkaline earth metals (Group IIA) form 2+ cations– for simple, monoatomic anions, use root of element name + “-ide” (ex. Cl– = chloride)Note: halides (Group VIIA) give 1– anionschalcogenides (Group VIA) give 2– anions
Type II: contains a metal ion that can form more than one kind of cation
• Transition metals can typically form more than one kind of cation(ex. Cu+, Cu2+ or Fe2+, Fe3+)
Rule: Indicate charge on cation using roman numerals (Stock system)
Examples:
2. Polyatomic Ions
Def: charged cluster of bonded atoms
Ex. NH4
+ = ammonium PO4
3- = phosphateOH– = hydroxide CO3
2- = carbonated. Oxoanions (or oxyanions)
Def: polyatomic ions containing “O”
Rule: Stem name plus a prefix and/or suffix denoting the relative number of oxygens
Note Trends:– If two members in a series, use “-ite” suffix for one with the fewest # of O atoms and “-ate” suffix for one with the most # of O atoms
Ex. SO3
2- = sulfite NO2
– = nitrite
SO4
2- = sulfate NO3
– = nitrate
– If more than two in a series, use the “hypo-” prefix for the fewest number of O atoms and “per-” for the most number of O atoms
Ex. CEO– = hypochlorite ClO2
– = chlorite
ClO3
– = chlorate ClO4
– = perchlorate
Examples:
3. Binary Molecular Compounds– contain typically two nonmetal atoms
Rules:– first element in formula named first– name the second element like anions– use prefixes (mono, di, tri, tetra, etc.) to denote #’s of atoms in the molecule
Note: Do not use “mono-” for naming the first element
Examples:
4. Hydrates
– contain weakly bound water molecules in its crystals- named from anhydrous compound (previous rules apply here)
Rule: Name by using Greek prefix for # of water molecules in formula and the word “hydrate” to denote water
Ex. Na2SO4 • 10H2O or Na2SO4 X 10 H2O(sodium sulfate decahydrate)
Acidic oxide
An acidic oxide is an oxide that either produces an acidic solution upon addition to water or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution (and the generation of carbonic acid) when dissolved.
The acidity of an oxide can be reasonably assumed by its accompanying constituents. Less electronegative elements tend to form basic oxides such as sodium oxide and magnesium oxide, whereas more electronegative elements tend to produce acidic oxides as seen with carbon dioxide and phosphorus pentoxide. Some oxides like aluminum oxides are amphoteric.
Acidic oxides are of environmental concern. Sulfur and nitrogen oxides are considered air pollutants as they react with atmospheric water vapor to produce acid rain.
permanganic acid
Permanganic acid (or manganic(VII) acid) is the inorganic compound with the formula HMnO4. This strong oxoacid has been isolated as its dihydrate. It is the conjugate acid of permanganate salts. It is the subject of few publications and its characterization as well as its uses are very limited.
Preparation and structure
Permanganic acid is most often prepared by the reaction of dilute sulfuric acid with a solution of barium permanganate, the insoluble barium sulfate byproduct being removed by filtering:
- Ba(MnO4)2 + H2SO4 → 2 HMnO4 + BaSO4↓
The sulfuric acid used must be dilute; reactions of permanganates with concentrated sulfuric acid yield the anhydride, manganese heptoxide.
Permanganic acid has also been prepared through the reaction of hydrofluorosilicic acid with potassium permanganate, through electrolysis, and through hydrolysis of manganese heptoxide, though the last route often results in explosions.
Crystalline permanganic acid has been prepared at low temperatures as the dihydrate, HMnO4·2H2O.
Although its structure has not been verified spectroscopically or crystallographically, HMnO4 is assumed to adopt a tetrahedral structure akin to that for perchloric acid.
Reactions
As a strong acid, HMnO4 is deprotonated to form the intensely purple-colored permanganates. Potassium permanganate, KMnO4, is a widely used, versatile, and powerful oxidizing agent.
Permanganic acid solutions are unstable and gradually decompose into manganese dioxide, oxygen, and water, with initially formed manganese dioxide catalyzing further decomposition. Decomposition is accelerated by heat, light, and acids. Concentrated solutions decompose more rapidly than dilute.