what causes capillary action
Hello. Welcome to solsarin. This post is about “what causes capillary action“.
Capillary action
Capillary action (sometimes called capillarity, capillary motion, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces like gravity. The effect can be seen in the drawing up of liquids between the hairs of a paint-brush, in a thin tube, in porous materials such as paper and plaster, in some non-porous materials such as sand and liquefied carbon fiber, or in a biological cell. It occurs because of intermolecular forces between the liquid and surrounding solid surfaces. If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces between the liquid and container wall act to propel the liquid.
Climb
Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and adhesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (picture to right) is limited by surface tension and, of course, gravity.
Not only does water tend to stick together in a drop, it sticks to glass, cloth, organic tissues, soil, and, luckily, to the fibers in a paper towel. Dip a paper towel into a glass of water and the water will “climb” onto the paper towel. In fact, it will keep going up the towel until the pull of gravity is too much for it to overcome.

Capillary action is all around us every day
- If you dip a paper towel in water, you will see it “magically” climb up the towel, appearing to ignore gravity. You are seeing capillary action in action, and “climbing up” is about right – the water molecules climb up the towel and drag other water molecules along. (Obviously, Mona Lisa is a big fan of capillary action!)
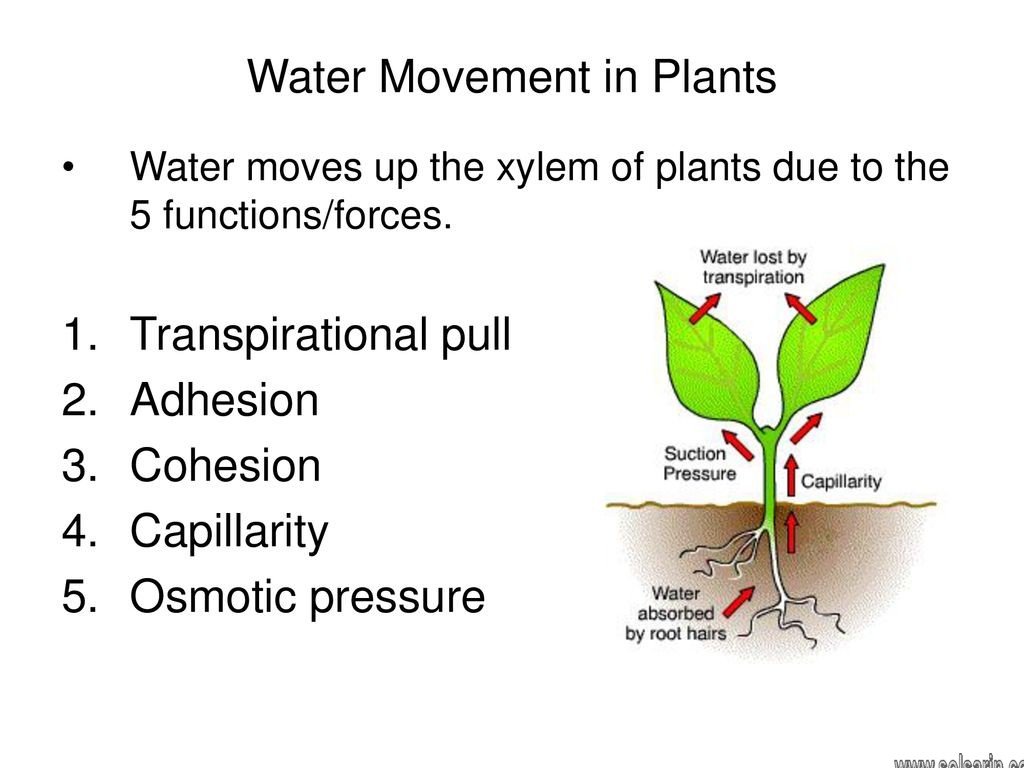
- Plants and trees couldn’t thrive without capillary action. Plants put down roots into the soil which are capable of carrying water from the soil up into the plant. Water, which contains dissolved nutrients, gets inside the roots and starts climbing up the plant tissue. Capillary action helps bring water up into the roots. But capillary action can only “pull” water up a small distance, after which it cannot overcome gravity. To get water up to all the branches and leaves, the forces of adhesion and cohesion go to work in the plant’s xylem to move water to the furthest leaf.
- Capillary action is also essential for the drainage of constantly produced tear fluid from the eye. Two tiny-diameter tubes, the lacrimal ducts, are present in the inner corner of the eyelid; these ducts secrete tears into the eye. (Source: Wikipedia)
- Maybe you’ve used a fountain pen …. or maybe your parents or grandparents did. The ink moves from a reservoir in the body of the pen down to the tip and into the paper (which is composed of tiny paper fibers and air spaces between them), and not just turning into a blob. Of course gravity is responsible for the ink moving “downhill” to the pen tip, but capillary action is needed to keep the ink flowing onto the paper.
Forces in Capillary Action
Three main variables that determine whether a liquid possesses capillary action are:
- Cohesive force: It is the intermolecular bonding of a substance where its mutual attractiveness forces them to maintain a certain shape of the liquid.
- Surface tension: This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in Newton/meter.
- Adhesive force: When forces of attraction between unlike molecules occur, it is called adhesive forces.
Capillary action only occurs when the adhesive forces are stronger than the cohesive forces, which invariably becomes surface tension, in the liquid.
A good way to remember the difference between adhesive and cohesive forces is that with adhesive forces you add another set of molecules, the molecules of the surface, for the liquid to bond with. With cohesive forces, the molecules of the liquid will only cooperate with their own kind. Decreased surface tension also increases capillary action. This is because decreased surface tension means that the intermolecular forces are decreased, thus decreasing cohesive forces. As a result, capillary action will be even greater.
Applications
Practical use of capillary action is evident in all forms of our daily lives. It makes performing our tasks efficiently and effectively. Some applications of this unique property include:
- The fundamental properties are used to absorb water by using paper towels. The cohesive and adhesive properties draw the fluid into the paper towel. The liquid flows into the paper towel at a certain rate.
- A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances.


- Capillary action helps us naturally by pumping out tear fluid in the eye. This process cleanses the eye and clears all of the dust and particles that are around the ducts of the eye.
- To generate energy: A possible use for capillary action is as a source of renewable energy. By allowing water to climb through capillaries, evaporate once it reaches the top, the condensate and drop back down to the bottom spinning a turbine on its way to create the energy, capillary action can make electricity! Although this idea is still in the works, it goes to show the potential that capillary action holds and how important it is.
- When measuring the level of liquid of a test tube or buret, it is imperative to measure at the meniscus line for an accurate reading. It is possible to measure the height (represented by h) of a test tube, buret, or other liquid column using the formula:
If you want to know about “what is the 2nd planet from the sun“, click on it.
In this formula,
- γ represents the surface tension in a liquid-air environment,
- θ is the angle of contact or the degree of contact,
- ρ is the density of the liquid in the representative column,
- g is the acceleration due to the force of gravity and
- r is the radius of the tube in which the liquid is presented in.
See Capillary Action Yourself
An excellent and easy demonstration of capillary action is done by placing a celery stalk in water. Color the water with food coloring and observe the progress of the dye up the celery stalk.
The same process may be used to color white carnations. Trim the bottom of a carnation stem to make sure it can absorb water. Place the flower in dyed water. The color will migrate via capillary action all the way to the flower petals.
A less dramatic but more familiar example of capillary action is the wicking behavior of a paper towel used to wipe up a spill.
Fast Facts: History of Capillary Action Study
- Capillary action was first recorded by Leonardo da Vinci.
- Robert Boyle performed experiments on capillary action in 1660, noting a partial vacuum had no effect on the height a liquid could obtain via wicking.
- A mathematical model of the phenomenon was presented by Thomas Young and Pierre-Simon Laplace in 1805.
- Albert Einstein’s first scientific paper in 1900 was written on the subject of capillarity.
Corrosionpedia Explains Capillary Action
Capillary action occurs because of intermolecular forces between the liquid and surrounding solid surfaces. It caused by the pressure of cohesion and adhesion, which cause the liquid to work against gravity.
The effect of capillary action can seen in:
- Drawing up of liquids between the hairs of a paintbrush
- Thin tubes
- Porous materials such as paper
- Some non-porous materials, such as liquefied carbon fiber
- Cells
Capillary action has many significant applications like thin layer chromatography, in which a solvent moves vertically up a plate via capillary action. The small pores of a sponge act as small capillaries, causing it to absorb a large amount of fluid. Some textile fabrics said to use capillary action to “wick” sweat away from the skin. These often referred to as wicking fabrics, after the capillary properties of candle and lamp wicks. In brazing, capillary action causes a filler metal to drawn into the space between workpieces.


Contributing Properties
Water is good at capillary action, better than most liquids. How well a liquid can perform the feat of capillary action depends on cohesion and adhesion. Cohesion is the attraction between particles of the same type. There is strong cohesion in water. One water molecule strongly attracted to another. Adhesion is the attraction between two different particles. The adhesion between water molecules and a plastic straw is also pretty strong.
Thank you for staying with this post “what causes capillary action” until the end.




