what is the charge of selenium
Hello. Welcome to solsarin. This post is about “what is the charge of selenium“.
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It rarely occurs in its elemental state or as pure ore compounds in the Earth’s crust. Selenium—from Greek selḗnē (σελήνη ‘Moon’)—was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium (named for the Earth).
If you want to know about “what is the photoelectric effect?“, click on it.
Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare.
DC power
The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been mostly replaced with silicon semiconductor devices. Selenium is still used in a few types of DC power surge protectors and one type of fluorescent quantum dot.


Although trace amounts of selenium are necessary for cellular function in many animals, including humans, both elemental selenium and (especially) selenium salts are toxic in even small doses, causing selenosis. Selenium is listed as an ingredient in many multivitamins and other dietary supplements, as well as in infant formula, and is a component of the antioxidant enzymes glutathione peroxidase and thioredoxin reductase (which indirectly reduce certain oxidized molecules in animals and some plants) as well as in 3 deiodinase enzymes. Selenium requirements in plants differ by species, with some plants requiring relatively large amounts and others apparently requiring none.
History
In 1817 Swedish chemist Jöns Jacob Berzelius noted a red substance resulting from sulfide ores from mines of Falun, Sweden. When this red material was investigated in the following year, it proved to be an element and was named after the Moon or the Moon goddess Selene. An ore of unusually high selenium content was discovered by Berzelius only days before he made his report to the scientific societies of the world on selenium. His sense of humour is evident in the name he gave the ore, eucairite, meaning “just in time.”
Occurrence and uses
The proportion of selenium in Earth’s crust is about 10−5 to 10−6 percent. It has been obtained mainly from the anode slimes (deposits and residual materials from the anode) in electrolytic refining of copper and nickel. Other sources are the flue dusts in copper and lead production and the gases formed in roasting pyrites. Selenium accompanies copper in the refining of that metal: about 40 percent of the selenium present in the original ore may concentrate in copper deposited in electrolytic processes. About 1.5 kilograms of selenium can be obtained from a ton of smelted copper.
When incorporated in small amounts into glass, selenium serves as a decolourizer; in larger quantities it imparts to glass a clear red colour that is useful in signal lights. The element is also employed in making red enamels for ceramics and steel ware, as well as for the vulcanization of rubber to increase resistance to abrasion.
Selenium refinement efforts are greatest in Germany, Japan, Belgium, and Russia.
Explanation:
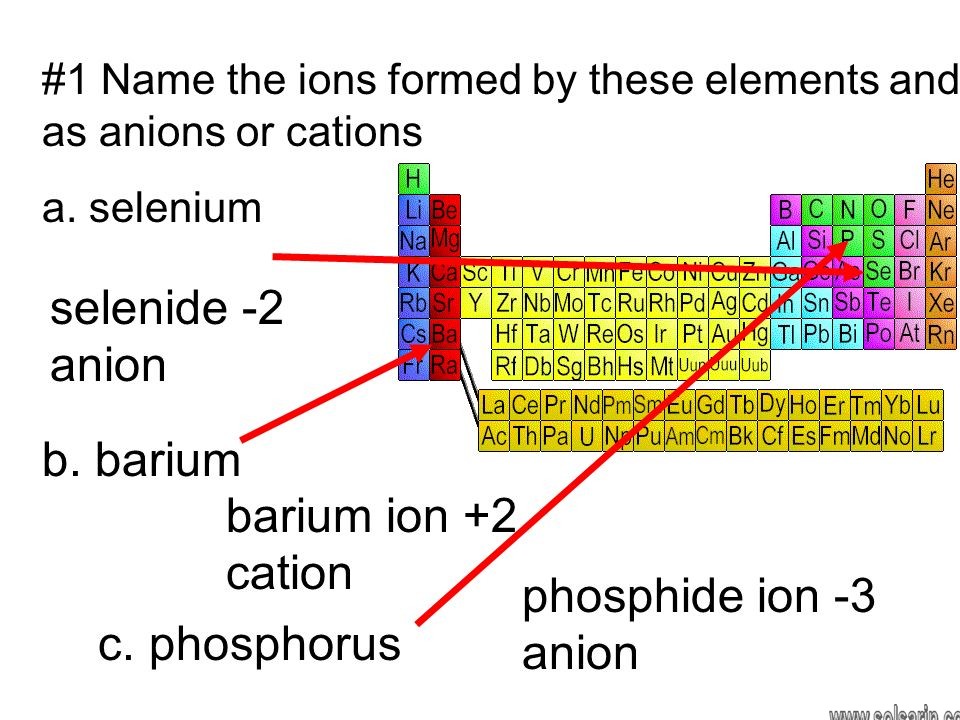
It would be easier for the element to become stable by accepting 2 electrons rather than donating 6 of its electrons. This suggests that the charge of the selenium ion must be −2 to undergo an ionic bond. Therefore, the charge on the ion that selenium forms in an ionic compound is −2 .
Selenium has the electronic configuration of . It’s easier for it to accept 2 valence electrons than donate its 6 valence electrons which requires higher energy. Therefore, the ion of selenium has the charge of 2-. It’s known as selenide ion.
Do you want to know about “how did marie curie discover radium” ? Click on it.
what is the charge of Al? The charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13 protons. The valence shell of aluminum has three electrons, and per the octet rule, these three electrons are lost resulting in just 10 electrons and 13 protons.
Considering this, is Selenium a positive or negative ion?
A Selenium is a nonmetal in group 16, so it will tend to gain electrons. B The nearest noble gas is krypton, so we predict that selenium will gain two electrons to form the Se2− ion, which has the same number of electrons as krypton.
What is selenide formula?
A selenide is a chemical compound containing a selenium anion with oxidation number of −2 (Se2−), much as sulfur does in a sulfide. The chemistry of the selenides and sulfides is similar. Similar to sulfide, in aqueous solution, the selenide ion, Se2−, is prevalent only in very basic conditions.
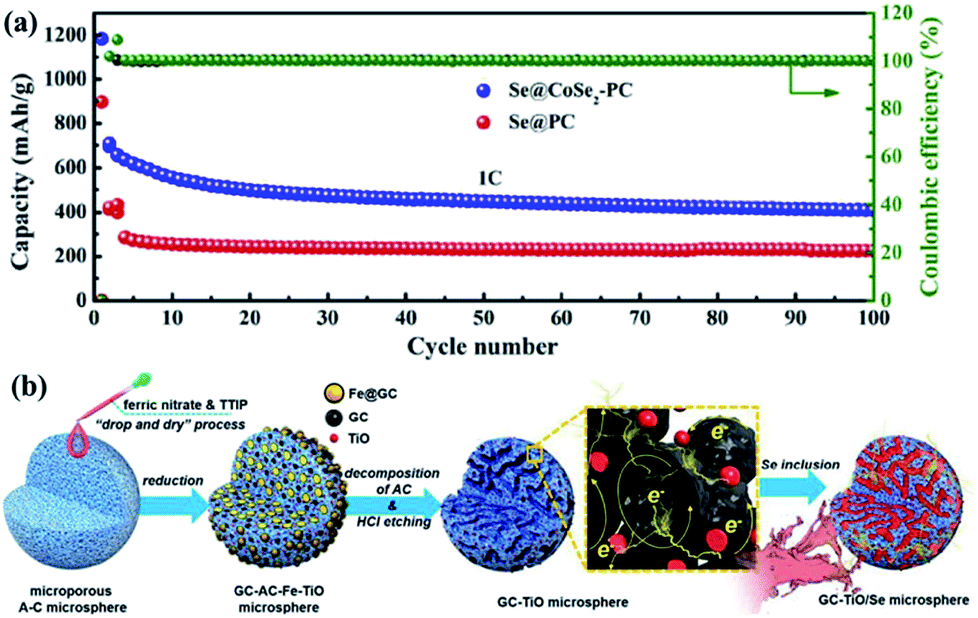

What Happened to the Se in the Marsh?
When scientists concluded that the wildlife casualties and deaths were the result of Se poisoning, the flow of waste water into the wetlands was stopped. However, water-soluble forms of Se are easily cycled through the environment. Plants take up Se through their roots, insects eat plants, and birds eat insects. Fish and their predators would also be exposed to Se. As long as there were substantial quantities of Se dissolved in the water, it would be difficult to prevent Se from entering the food chain.
The solubility of Se varies, depending on its form. Selenium has three common forms:
- Selenate, a water-soluble anion.
- Selenite, a virtually water-insoluble anion.
- Elemental Se, a water-insoluble crystal.
What is the symbol and charge for selenium?
Selenium(4+) | Se+4 – PubChem.
What kind of ion does selenium form?
Therefore, the ion of selenium has the charge of 2-. It’s known as selenide ion….What is a selenium ion called?
PubChem CID : 107674
What anion will selenium form?
Selenium (Se) has an atomic number, and belongs to group 6A. So it will have 6 valence electrons. When two electrons are gained by selenium atom, it forms an anion having charge of -2.
selenium has 6 valance electrons, therefore in order to be electronically stable, it wants to gain two electrons, in order to have a full shell of 8 valance electrons.
when an atom gain electrons, it becomes negatively charged, therefore the charge is 2-
selenium is in group 6A and is a cousin to sulfur.Itexhibits the -2 charge as selenide ( b). It can aslo , like sulfur form oxyacids such as selenic acid ans selenous acid, H2SeO4 and H2SeO3 respectively in which its oxidation states are +6 and +4 respectively.In his case the only answer that is correct is b -2 like in Na2 Se sodium selenide or hydrogen selenide
Atomic Mass of Selenium
Atomic mass of Selenium is 78.96 u.
The atomic mass is the mass of an atom. Relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
Atomic Radius of Selenium
The atomic radius of Selenium atom is 120pm (covalent radius).
It must be noted, atoms lack a well-defined outer boundary. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Therefore, there are various non-equivalent definitions of atomic radius.
Electrons and Electron Configuration
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Selenium is 34. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Have you heard anything about “belt between the orbits of mars and jupiter” ? Click on it.
Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
Electron configuration of Selenium is [Ar] 3d10 4s2 4p4.
Possible oxidation states are +4,6/-2.
What does an ion that has a charge of +2 mean?
If an ion has a 2+ charge then it must have lost electrons to form the cation. If the ion has 18 electrons and the atom lost 2 to form the ion, then the neutral atom contained 20 electrons. Since it was neutral, it must also have had 20 protons. Therefore the element is calcium.

What is the charge of CL most common ion?
1− charge
What is the normal phase of selenium?
Solid
What are the signs of selenium deficiency?
What are the symptoms?
- infertility in men and women.
- muscle weakness.
- fatigue.
- mental fog.
- hair loss.
- weakened immune system.
Thank you for staying with this post “what is the charge of selenium” until the end.




