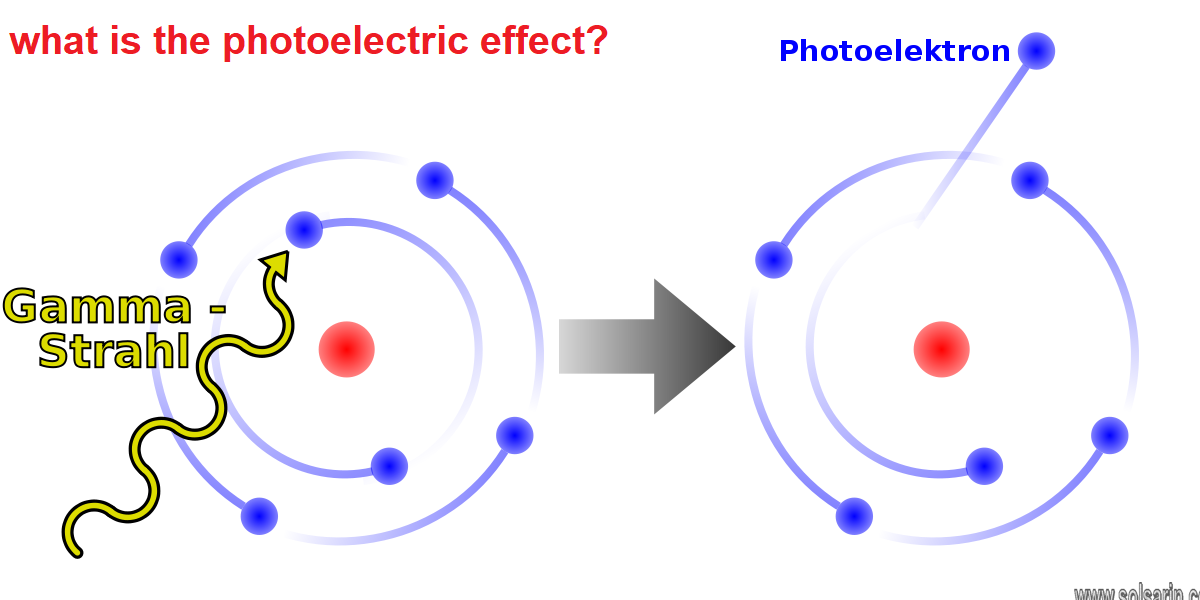
what is the photoelectric effect?
Hello. Welcome to solsarin. This post is about “what is the photoelectric effect?“.
Photoelectric effect
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.
The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the light’s intensity or duration of exposure.
Albert Einstein
Because a low-frequency beam at a high intensity could not build up the energy required to produce photoelectrons, as it would have if light’s energy were coming from a continuous wave, Albert Einstein proposed that a beam of light is not a wave propagating through space, but a swarm of discrete energy packets, known as photons.
If you want to know about “how did marie curie discover radium“, click on it.
Emission of conduction electrons from typical metals requires a few electron-volt (eV) light quanta, corresponding to short-wavelength visible or ultraviolet light. In extreme cases, emissions are induced with photons approaching zero energy, like in systems with negative electron affinity and the emission from excited states, or a few hundred keV photons for core electrons in elements with a high atomic number. Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality. Other phenomena where light affects the movement of electric charges include the photoconductive effect, the photovoltaic effect, and the photoelectrochemical effect.
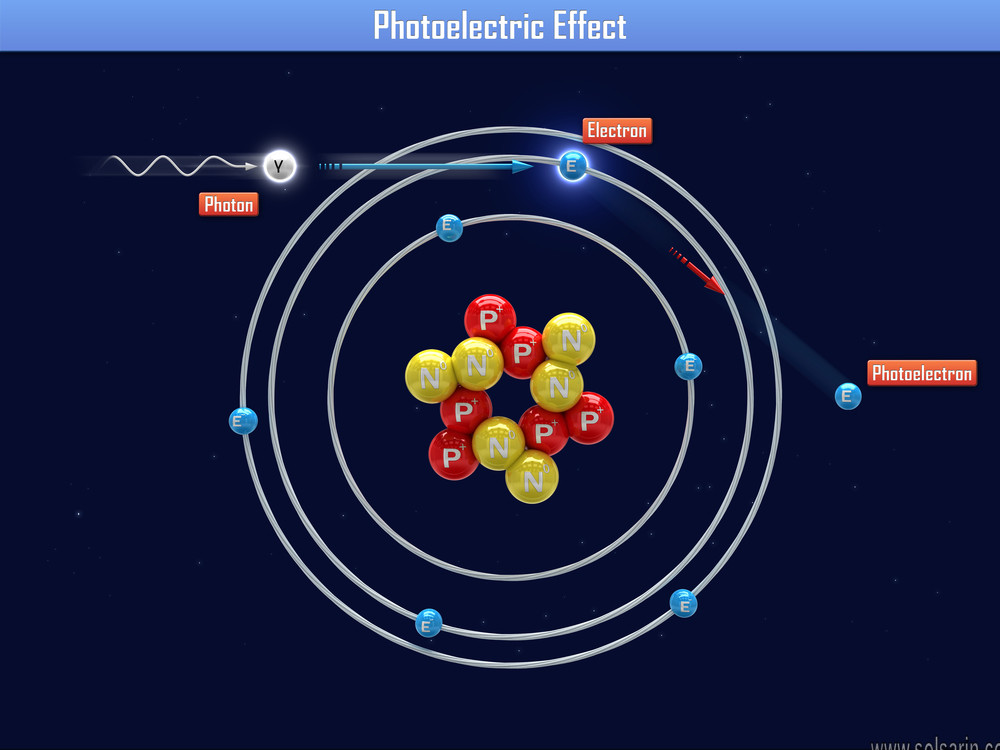

Discovery and early work
The photoelectric effect was discovered in 1887 by the German physicist Heinrich Rudolf Hertz. In connection with work on radio waves, Hertz observed that, when ultraviolet light shines on two metal electrodes with a voltage applied across them, the light changes the voltage at which sparking takes place. This relation between light and electricity (hence photoelectric) was clarified in 1902 by another German physicist, Philipp Lenard. He demonstrated that electrically charged particles liberated from a metal surface when it illuminated and that these particles are identical to electrons, which discovered by the British physicist Joseph John Thomson in 1897.
Further research showed that the photoelectric effect represents an interaction between light and matter that cannot explained by classical physics, which describes light as an electromagnetic wave. One inexplicable observation was that the maximum kinetic energy of the released electrons did not vary with the intensity of the light, as expected according to the wave theory, but was proportional instead to the frequency of the light. What the light intensity did determine was the number of electrons released from the metal (measured as an electric current). Another puzzling observation was that there was virtually no time lag between the arrival of radiation and the emission of electrons.
1905
Consideration of these unexpected behaviours led Albert Einstein to formulate in 1905 a new corpuscular theory of light in which each particle of light, or photon, contains a fixed amount of energy, or quantum, that depends on the light’s frequency. In particular, a photon carries an energy E equal to hf, where f is the frequency of the light and h is the universal constant that the German physicist Max Planck derived in 1900 to explain the wavelength distribution of blackbody radiation—that is, the electromagnetic radiation emitted from a hot body. The relationship may also written in the equivalent form E = hc/λ, where c is the speed of light and λ is its wavelength, showing that the energy of a photon is inversely proportional to its wavelength.
Do you want to know about “what is a thrust fault” ? Click on it.
Einstein assumed that a photon would penetrate the material and transfer its energy to an electron. As the electron moved through the metal at high speed and finally emerged from the material, its kinetic energy would diminish by an amount ϕ called the work function (similar to the electronic work function), which represents the energy required for the electron to escape the metal. By conservation of energy, this reasoning led Einstein to the photoelectric equation Ek = hf − ϕ, where Ek is the maximum kinetic energy of the ejected electron.
1921
Although Einstein’s model described the emission of electrons from an illuminated plate, his photon hypothesis was sufficiently radical that it not universally accepted until it received further experimental verification. Further corroboration occurred in 1916 when extremely accurate measurements by the American physicist Robert Millikan verified Einstein’s equation and showed with high precision that the value of Einstein’s constant h was the same as Planck’s constant. Einstein finally awarded the Nobel Prize for Physics in 1921 for explaining the photoelectric effect.
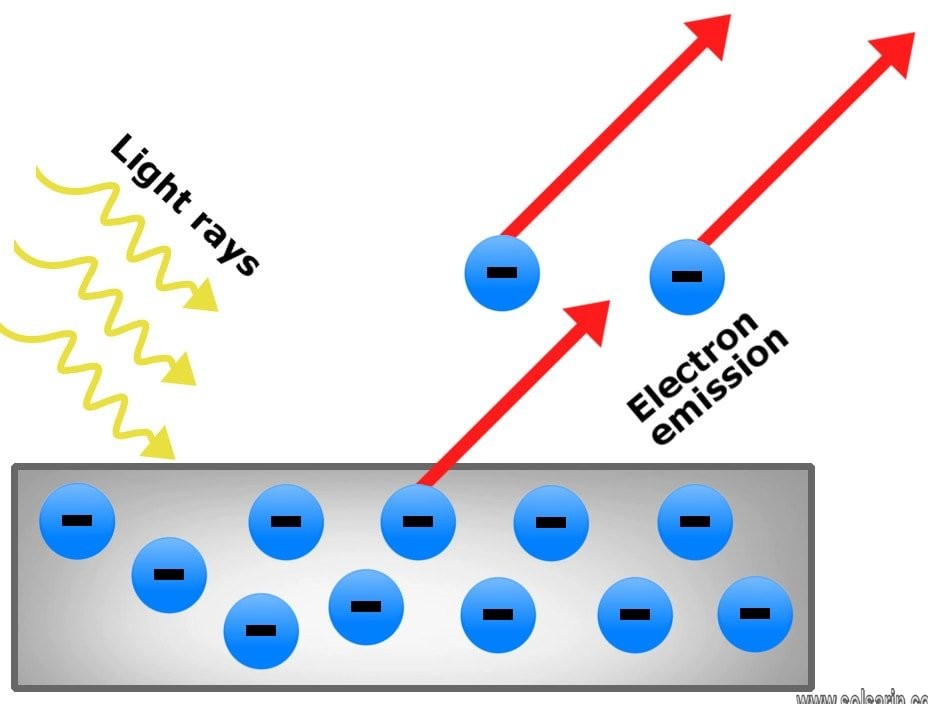

In 1922 the American physicist Arthur Compton measured the change in wavelength of X-rays after they interacted with free electrons, and he showed that the change could calculated by treating X-rays as made of photons. Compton received the 1927 Nobel Prize for Physics for this work. In 1931 the British mathematician Ralph Howard Fowler extended the understanding of photoelectric emission by establishing the relationship between photoelectric current and temperature in metals. Further efforts showed that electromagnetic radiation could also emit electrons in insulators, which do not conduct electricity, and in semiconductors, a variety of insulators that conduct electricity only under certain circumstances.
Predictions based on light as a wave
Based on the classical description of light as a wave, they made the following predictions:
-
The kinetic energy of emitted photoelectrons should increase with the light amplitude.
-
The rate of electron emission, which proportional to the measured electric current, should increase as the light frequency increased.
Have you heard anything about “first antibiotic discovered” ? Click on it.
Applications
While the description of the photoelectric effect sounds highly theoretical, there are many practical applications of its work. Britannica describes a few:
Photoelectric cells originally used to detect light, using a vacuum tube containing a cathode, to emit electrons, and an anode, to gather the resulting current. Today, these “phototubes” have advanced to semiconductor-based photodiodes that used in applications such as solar cells and fiber optics telecommunications.
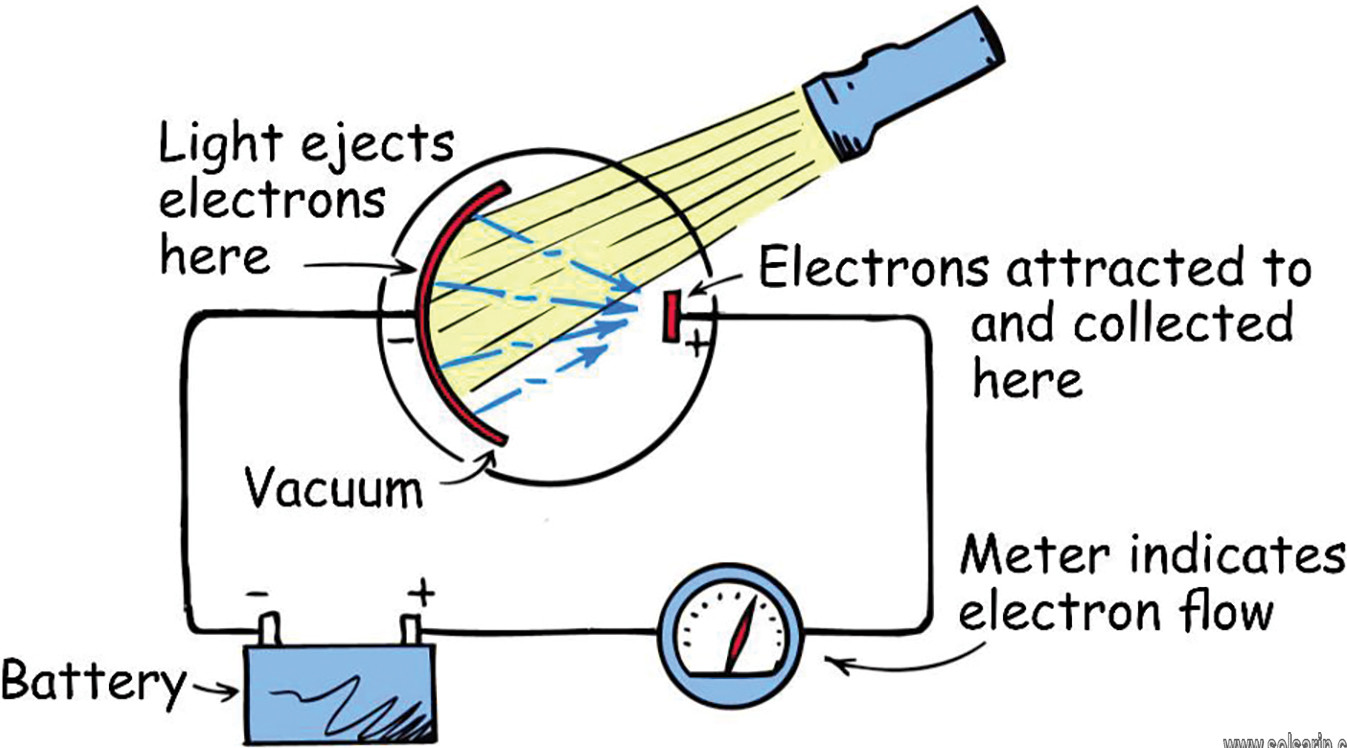

Photomultiplier tubes are a variation of the phototube, but they have several metal plates called dynodes. Electrons released after light strikes the cathodes. The electrons then fall onto the first dynode, which releases more electrons that fall on the second dynode, then on to the third, fourth, and so forth. Each dynode amplifies the current; after about 10 dynodes, the current is strong enough for the photomultipliers to detect even single photons. Examples of this used in spectroscopy (which breaks apart light into different wavelengths to learn more about the chemical compositions of star, for example), and computerized axial tomography (CAT) scans that examine the body.
Other applications of photodiodes and photomultipliers include:
- imaging technology, including (older) television camera tubes or image intensifiers;
- studying nuclear processes;
- chemically analyzing materials based on their emitted electrons;
- giving theoretical information about how electrons in atoms transition between different energy states.
But perhaps the most important application of the photoelectric effect was setting off the quantum revolution, according to Scientific American. It led physicists to think about the nature of light and the structure of atoms in an entirely new way.
Thank you for staying with this post “what is the photoelectric effect?” until the end.
More Posts :