what links amino acids together
Welcom to solsarin site ,Keep reading and find the answer about “what links amino acids together ”.
Stay with us.
Thank you for your support.
amino acid
amino acid, any of a group of organic molecules that consist of a basic amino group (―NH2), an acidic carboxyl group (―COOH), and an organic R group (or side chain) that is unique to each amino acid. The term amino acid is short for α-amino [alpha-amino] carboxylic acid. Each molecule contains a central carbon (C) atom, called the α-carbon, to which both an amino and a carboxyl group are attached. The remaining two bonds of the α-carbon atom are generally satisfied by a hydrogen (H) atom and the R group.

Chirality
All the amino acids but glycine are chiral molecules. That is, they exist in two optically active asymmetric forms (called enantiomers) that are the mirror images of each other.
(This property is conceptually similar to the spatial relationship of the left hand to the right hand.)
One enantiomer is designated D and the other L.
It is important to note that
It is important to note that the amino acids found in proteins almost always possess only the L-configuration. This reflects the fact that the enzymes responsible for protein synthesis have evolved to utilize only the L-enantiomers.
Reflecting this near universality, the prefix L is usually omitted.
Some D-amino acids are found in microorganisms, particularly in the cell walls of bacteria and in several of the antibiotics. However, these are not synthesized in the ribosome.
Why are there 20 different types of amino acids?
The decisive factor is the greater chemical reactivity of the newer amino acids rather than their spatial structure.
In the inherited DNA, it is always three sequential DNA bases, or codons,
which combine to “encode” one single of these 20 amino acids.
The resultant grid of codons is what is known as the genetic code.
How many amino acids are there in humans?
Roughly 500 amino acids have been identified in nature, but just 20 amino acids make up the proteins found in the human body.
Non essential amino acids
Nonessential means that our bodies can produce the amino acid, even if we do not get it from the food we eat.
Nonessential amino acids include: alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.

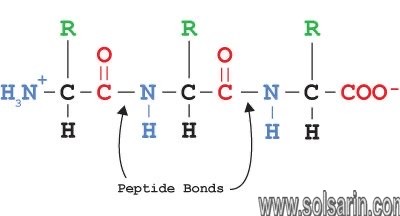
Peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.
How peptide bond is formed?
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O).
This is a dehydration synthesis reaction (also known as a condensation reaction), and usually occurs between amino acids.
What are the characteristics of a peptide bond?
A peptide bond is a planar, trans and rigid configuration.
It also shows a partial double bond character.
The coplanarity of the peptide bond denotes the resonance or partial sharing of two pairs of electrons between the amide nitrogen and carboxyl oxygen.
What are proteins?
Proteins are the end products of the decoding process that starts with the information in cellular DNA.
As workhorses of the cell, proteins compose structural and motor elements in the cell, and they serve as the catalysts for virtually every biochemical reaction that occurs in living things.
This incredible array of functions derives from a startlingly simple code that specifies a hugely diverse set of structures.

In fact
In fact, each gene in cellular DNA contains the code for a unique protein structure.
Not only are these proteins assembled with different amino acid sequences,
but they also are held together by different bonds and folded into a variety of three-dimensional structures.
The folded shape, or conformation, depends directly on the linear amino acid sequence of the protein.
What Are Proteins Made Of?
The building blocks of proteins are amino acids, which are small organic molecules that consist of an alpha (central) carbon atom linked to an amino group, a carboxyl group, a hydrogen atom, and a variable component called a side chain (see below).

Proteins are built from
Proteins are built from a set of only twenty amino acids, each of which has a unique side chain.
The side chains of amino acids have different chemistries.
The largest group of amino acids have nonpolar side chains. Several other amino acids have side chains with positive or negative charges, while others have polar but uncharged side chains.
The chemistry of amino acid side chains is critical to protein structure because these side chains can bond with one another to hold a length of protein in a certain shape or conformation.
Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. Hydrophobic side chains interact with each other via weak van der Waals interactions.
The vast majority of bonds formed by these side chains are noncovalent.
In fact, cysteines are the only amino acids capable of forming covalent bonds, which they do with their particular side chains. Because of side chain interactions, the sequence and location of amino acids in a particular protein guides where the bends and folds occur in that protein
The primary structure
The primary structure of a protein — its amino acid sequence — drives the folding and intramolecular bonding of the linear amino acid chain, which ultimately determines the protein’s unique three-dimensional shape.
Hydrogen bonding between amino groups and carboxyl groups in neighboring regions of the protein chain sometimes causes certain patterns of folding to occur. Known as alpha helices and beta
sheets, these stable folding patterns make up the secondary structure of a protein.
Most proteins contain multiple helices and sheets, in addition to other less common patterns .
The ensemble of formations and folds in a single linear chain of amino acids — sometimes called a polypeptide — constitutes the tertiary structure of a protein. Finally, the quaternary structure of a protein refers to those macromolecules with multiple polypeptide chains or subunits.
The final shape
The final shape adopted by a newly synthesized protein is typically the most energetically favorable one.
As proteins fold, they test a variety of conformations before reaching their final form,
which is unique and compact. Folded proteins are stabilized by thousands of noncovalent bonds between amino acids. In addition, chemical forces between a protein and its immediate environment contribute to protein shape and stability.
fully folded proteins are not frozen into shape
It is important to note, however, that fully folded proteins are not frozen into shape. Rather, the atoms within these proteins remain capable of making small movements.
Even though proteins are considered macromolecules, they are too small to visualize, even with a microscope. So, scientists must use indirect methods to figure out what they look like and how they are folded.
The most common method used to study protein structures is X-ray crystallography.
With this method, solid crystals of purified protein are placed in an X-ray beam, and the pattern of deflected X rays is used to predict the positions of the thousands of atoms within the protein crystal.
How Do Proteins Arrive at Their Final Shapes?
In theory, once their constituent amino acids are strung together, proteins attain their final shapes without any energy input.
In reality, however, the cytoplasm is a crowded place, filled with many other macromolecules capable of interacting with a partially folded protein. Inappropriate associations with nearby proteins can interfere with proper folding and cause large aggregates of proteins to form in cells. Cells therefore rely on so-called chaperone proteins to prevent these inappropriate associations with unintended folding partners.
Chaperone proteins
Chaperone proteins surround a protein during the folding process, sequestering the protein until folding is complete.
For example, in bacteria, multiple molecules of the chaperone GroEL form a hollow chamber around proteins that are in the process of folding.
Molecules of a second chaperone, GroES, then form a lid over the chamber.
Eukaryotes use different families of chaperone proteins, although they function in similar ways.
Chaperone proteins are abundant in cells.
Chaperone proteins are abundant in cells.
These chaperones use energy from ATP to bind and release polypeptides as they go through the folding process.
Chaperones also assist in the refolding of proteins in cells.
Folded proteins are actually fragile structures, which can easily denature, or unfold.
Although many thousands of bonds hold proteins together,
most of the bonds are noncovalent and fairly weak. Even under normal circumstances, a portion of all cellular proteins are unfolded. Increasing body temperature by only a few degrees can significantly increase the rate of unfolding. When this happens, repairing existing proteins using chaperones is much more efficient than synthesizing new ones. Interestingly, cells synthesize additional chaperone proteins in response to “heat shock.”
What are the 10 essential amino acids for humans?
These are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine.
Random posts



