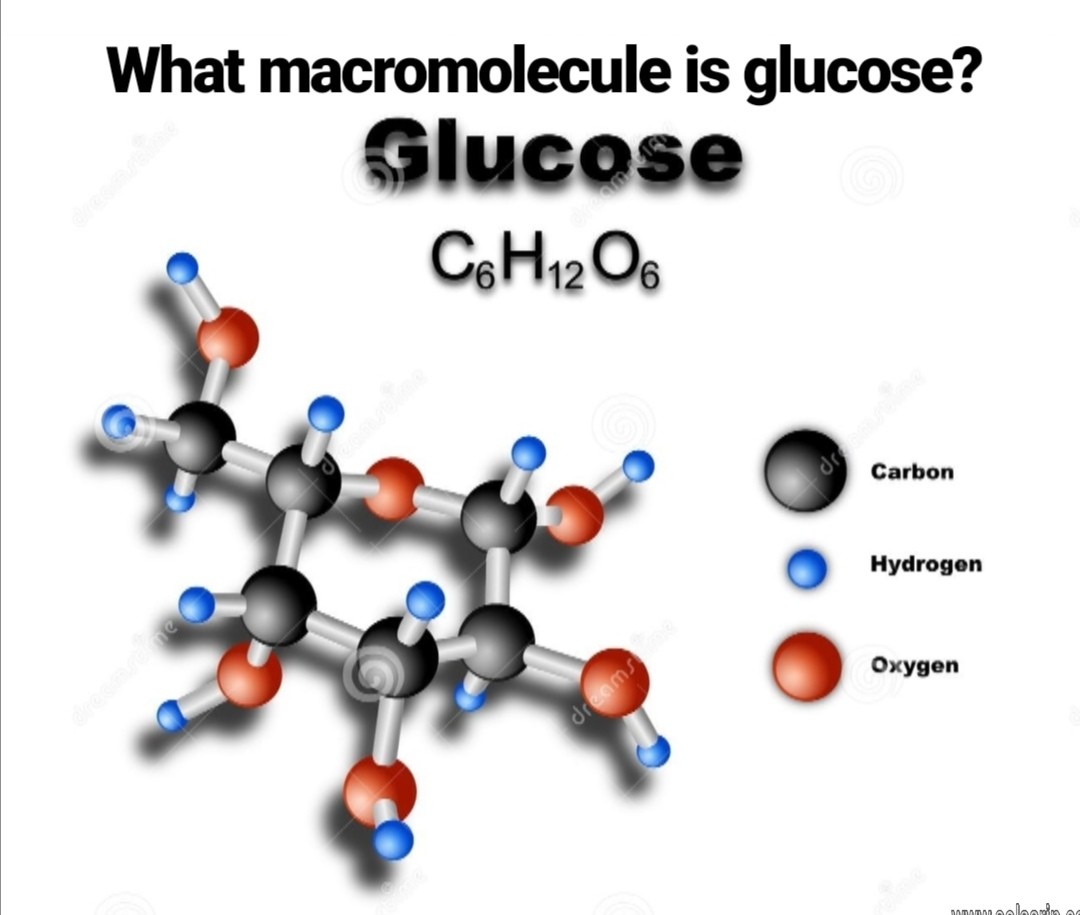
what macromolecule is glucose?
Hi, welcome to solsarin site, in this post we want to talk about“what macromolecule is glucose”,
thank you for choosing us.
what macromolecule is glucose?
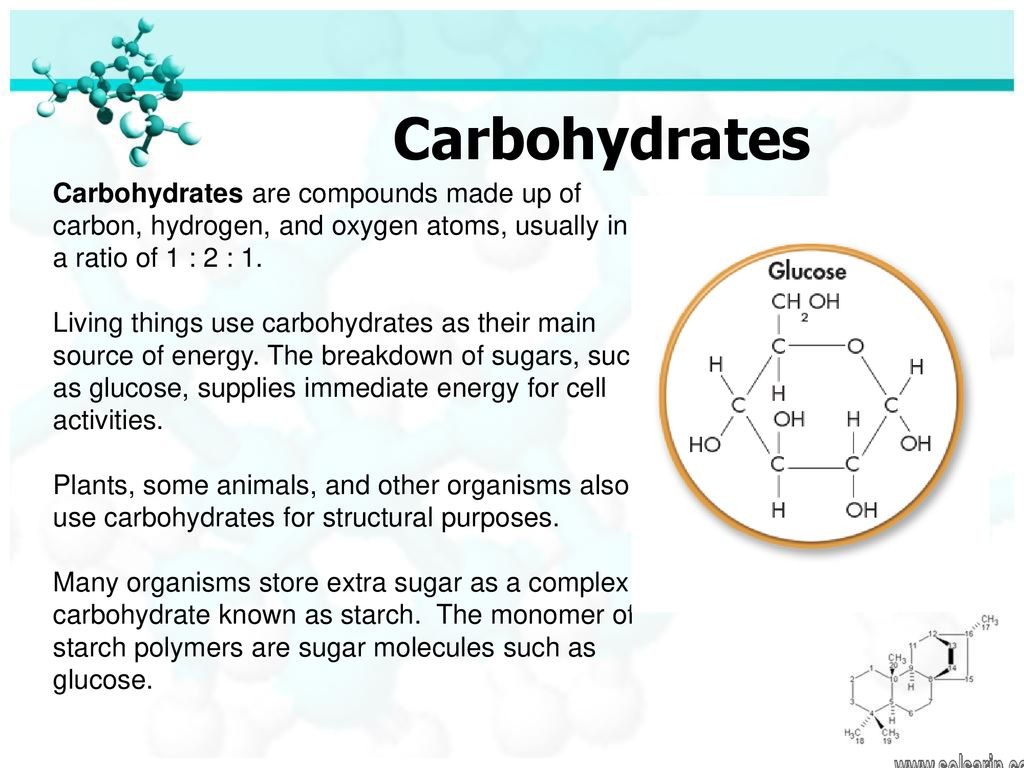
Glucose is a carbohydrate with the molecular formula C6H12O6, specifically a monosaccharide. This is like saying peas are a vegetable, and more specifically a legume–a type of vegetable. Being even more specific, glucose is a simple sugar. It is variably called grape sugar, blood sugar and corn sugar.
What type of macromolecule is glucose?
Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. Glucose is a carbohydrate, and is the most important simple sugar in human metabolism.

Carbohydrates
Carbohydrates are the most abundant of the major macromolecules found in the human body. The other three are nucleic acids, proteins and lipids. Carbohydrates are involved with the storage and transportation of energy.
Carbohydrates are the sugars and their polymers. Simple sugars are called monosaccharides. Glucose is an important monosaccharide. Sucrose, a disaccharide (consisting of two monosaccharides), is table sugar. (Note the ending “ose” common to most sugars.)
Polysaccharides may be made from thousands of simple sugars linked together. These large molecules may be used for storage of energy or for structure. First a couple of storage examples:
Starch is a storage polysaccharide of plants. Its is a giant string of glucoses. The plant can utilize the energy in starch by first hydrolyzing it, making the glucose available. Most animals can also hydrolyze starch. That’s why we eat it.
Animals store glycogen as a supply of glucose.
And some examples of structural carbohydrates:
Cellulose is a polysaccharide produced by plants. Its is a component of the cell walls. Cellulose is also a string of glucose molecules. Because the glucoses are joined together differently cellulose has a different shape, and therefor different properties, than starch or glycogen. The enzymes (we’ll learn more about these soon) that are used to hydrolyze starch don’t work on cellulose. Most organisms cannot digest cellulose and it passes right through them (roughage). Goats and termites don’t really digest cellulose, they have bacteria that do it for them.
Monosaccharides
Monosaccharides are the simplest form of carbohydrate: simple sugars. They can not be broken down into simpler carbohydrates. Monosaccharides can be linked together to form disaccharides or even polysaccharides.

Types of biological macromolecules
| Biological macromolecule | Building blocks | Functions | Examples |
|---|---|---|---|
| Carbohydrates | Monosaccharides (simple sugars) | Provide cells with quick/short-term energy, source of dietary fiber | Glucose, sucrose, starch, cellulose, chitin |
| Lipids | Fatty acids and glycerol | Provide cells with long-term energy, make up biological membranes | Fats, phospholipids, waxes, oils, grease, steroids |
| Proteins | Amino acids | Provide cell structure, send chemical signals, speed up chemical reactions, etc | Keratin (found in hair and nails), hormones, enzymes, antibodies |
| Nucleic acids | Nucleotides | Store and pass on genetic information | DNA, RNA |
Monomers and polymers
Lipids
Lipids include a diverse group of compounds that are united by a common feature. they are
hydrophobic (“water-fearing”), or insoluble in water, because they are nonpolar molecules. This is because they are hydrocarbons that include only nonpolar carbon-carbon or carbon-hydrogen bonds. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of lipids called fats.
Lipids also provide insulation from the environment for plants and animals. For example, they help keep aquatic birds and mammals dry because of their water-repelling nature. Lipids are also the building blocks of many hormones and are an important constituent of the plasma membrane. Lipids include fats, oils, waxes, phospholipids, and steroids.
A fat molecule, such as a triglyceride, consists of two main components—glycerol and fatty acids.Moreover, Glycerol is an organic compound with three carbon atoms, five hydrogen atoms, and three hydroxyl (–OH) groups. Fatty acids have a long chain of hydrocarbons to which an acidic carboxyl group is attached, hence the name “fatty acid.” The number of carbons in the fatty acid may range from 4 to 36; most common are those containing 12–18
carbons. In a fat molecule, a fatty acid is attached to each of the three oxygen atoms in the –OH groups of the glycerol molecule with a covalent bond.

Amino acids polymerize to form polypeptides or proteins.
Amino acids contain a carboxylic acid (-COOH) group and an amino (-NH2) group. The amino groups are usually attached to the carbons which are alpha to the carboxyl carbons,
so they are called alpha-amino acids.
The naturally occurring amino acids are optically active, as they have four different groups attached to one carbon, (Glycine is an exception, having two hydrogens) and have the L-configuration.
The R-groups of the amino acids provide a basis for classifying amino acids. There are many ways of classifying amino acids, but one very useful way is on the basis of how well or poorly the R-group interacts with water
- The first class is the hydrophobic R-groups which can be aliphatic (such as the methyl group of alanine) or aromatic (such as the phenyl group of phenylalanine).
- The second class is the hydrophilic R-groups which can contain neutral polar (such as the -OH of serine) or ionizable (such as the -COOH of aspartate) functional groups.
Amino acids polymerize by eliminating the elements of water
to form an amide between the amino and carboxyl groups.
The product has ends with different properties.
- An end with a free amino group; this is called the amino terminal or N-terminal.
- An end with a free carboxyl group; this is called the carboxyl terminal or C-terminal.
Conventions for writing sequences of amino acids.
Abbreviations for the amino acids are usually used; most of the three letter abbreviations are self-evident, such as gly for glycine, asp for aspartate, etc.
There is also a one-letter abbreviation system; it is becoming more common. Many of the one-letter abbreviations are straightforward, for example:
- G = glycine
- L = leucine
- H = histidine
Others require a little imagination to justify:
- F = phenylalanine (“ph” sounds like “F”).
- Y = tyrosine (T was used for threonine, so we settle for the second letter in the name).
- D = aspartate (D is the fourth letter in the alphabet, and aspartate has four carbons).
Still others are rather difficult to justify:
- W = tryptophan (The bottom half of the two aromatic rings look sort of like a “W”).
- K = lysine (if you can think of a good one for this, let us know!)
Question: What do you suppose “Q” represents?
You should be aware this is becoming more and more commonly used, and you should have
the mindset of picking it up as you are exposed to it, rather than resisting.
Although R-groups of some amino acids contain amino and carboxyl groups, branched polypeptides or proteins do not occur.
The sequence of monomer units in a macromolecule is called the PRIMARY STRUCTURE of that macromolecule. Each specific macromolecule has a unique primary structure.
This concludes our consideration of the relationship between the structures of biological polymers and their monomer subunits. Let’s now begin to investigate the three-dimensional shapes of these macromolecules in solution and the forces responsible for these shapes.
Nucleic Acids
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are polymers of nucleotides (3.20a, pg 47). Later we will learn in more detail the roles these nucleic acids play in protein synthesis.
Nucleotides are made of three parts: a phosphate, a pentose sugar, and a nitrogenous base. The pentose sugar of DNA is deoxyribose. The pentose sugar of RNA is ribose.
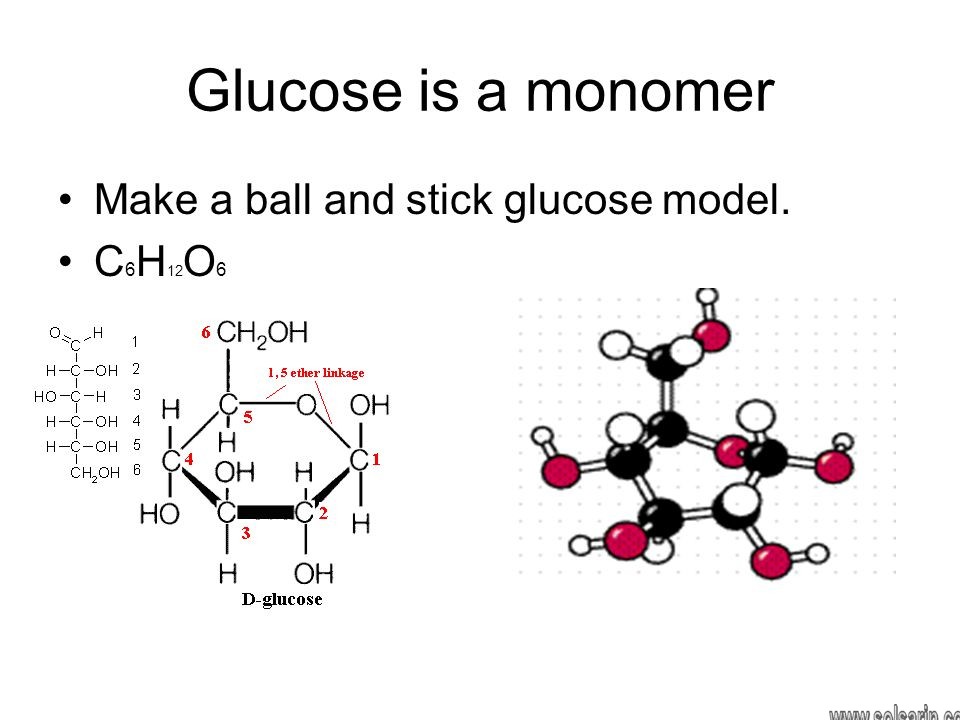

What macromolecule is glucose and starch?
Carbohydrates. Carbohydrates include simple sugars (monosaccharides) as well as large polymers (polysaccharides).Moreover, Glucose is a hexose, a sugar composed of six carbon atoms, usually found in ring form.
What are the 4 macromolecules?
There are four major classes of biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids); each is an important cell component and performs a wide array of functions.
Which type of macromolecule is glucose & What is its function?
The monosaccharides bond together to form polysaccharides, which are the polymers of carbohydrates. Moreover, The most common monosaccharide is glucose, which is one of the most valuable sugars for all animals and plants. The function of carbohydrates is to act as an energy source for storage and structure for all living things.
Is glucose a carbohydrate?
The two main forms of carbohydrates are: sugars such as fructose, glucose, and lactose. starches, which are found in foods such as starchy vegetables (like potatoes or corn), grains,
rice, breads, and cereals.
MORE POSTS: