what number is krypton in the periodic table?
Hi dear friends, solsarin in this article is talking about “what number is krypton in the periodic table?”.
we are happy to have you on our website.

krypton (Kr), chemical element, a rare gas of Group 18 (noble gases) of the periodic table, which forms relatively few chemical compounds. About three times heavier than air, krypton is colourless, odourless, tasteless, and monatomic. Although traces are present in meteorites and minerals, krypton is more plentiful in Earth’s atmosphere, which contains 1.14 parts per million by volume of krypton. The element was discovered in 1898 by the British chemists Sir William Ramsay and Morris W. Travers in the residue left after a sample of liquid air had boiled almost entirely away.
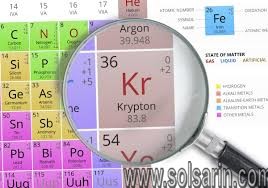
Element Propertiesatomic number36atomic weight83.798melting point−157.4 °C (−251.3 °F)boiling point−153.4 °C (−244.1 °F)density (1 atm, 0 °C [32 °F])3.733 g/litre (0.049 ounce/gallon)oxidation numbers0, 2electron configuration(Ar)3d104s24p6
History
From the Greek word kryptos, hidden. Discovered in 1898 by Ramsay and Travers in the residue left after liquid air had nearly boiled away. In 1960 it was internationally agreed that the fundamental unit of length, the meter, should be defined in terms of the orange-red spectral line of 86Kr. This replaced the standard meter of Paris, which was defined in terms of a bar made of a platinum-iridium alloy. In October 1983, the meter, which originally was defined as being one ten millionth of a quadrant of the earth’s polar circumference, was again redefined by the International Bureau of Weights and Measures as being the length of a path traveled by light in a vacuum during a time interval of 1/299,792,458 of a second.
Sources
Krypton is present in the air to the extent of about 1 ppm. The atmosphere of Mars has been found to contain 0.3 ppm of krypton. Solid krypton is a white crystalline substance with a face-centered cubic structure which is common to all the “rare gases.”
Properties
Krypton is a “noble” gas. It is characterized by its brilliant green and orange spectral lines.
Isotopes
Naturally occurring krypton contains six stable isotopes. Seventeen other unstable isotopes are recognized. The spectral lines of krypton are easily produced and some are very sharp. While krypton is generally thought of as a rare gas that normally does not combine with other elements to form compounds, it now appears that the existence of some krypton compounds can exist. Krypton difluoride has been prepared in gram quantities and can be made by several methods. A higher fluoride of krypton and a salt of an oxyacid of krypton also have been reported. Molecule-ions of ArKr+ and KrH+ have been identified and investigated, and evidence is provided for the formation of KrXe or KrXe+.
Uses
Krypton clathrates are prepared using hydroquinone and phenol. 85Kr can be used for chemical analysis by imbedding the isotope in various solids. During this process, kryptonates are formed. Kryptonate activity is sensitive to chemical reactions at the solution surface. Estimates of the concentration of reactants are therefore made possible. Krypton is used in certain photographic flash lamps for high-speed photography.
| atomic number | 36 |
|---|---|
| atomic weight | 83.798 |
| melting point | −157.4 °C (−251.3 °F) |
| boiling point | −153.4 °C (−244.1 °F) |
| density (1 atm, 0 °C [32 °F]) | 3.733 g/litre (0.049 ounce/gallon) |
| oxidation numbers | 0, 2 |
| electron configuration | (Ar)3d104s24p6 |
Properties of the element


Because its boiling point (−153.4 °C, or −244.1 °F) is about 30–40 °C (50–70 °F) higher than those of the major constituents of air, krypton is readily separated from liquid air by fractional distillation; it accumulates along with xenon in the least volatile portion. These two gases are further purified by adsorption onto silica gel, redistillation, and passage over hot titanium metal, which removes all impurities except other noble gases.
Krypton is used in certain electric and fluorescent lamps and in a flashlamp employed in high-speed photography. Radioactive krypton-85 is useful for detecting leaks in sealed containers, with the escaping atoms detected by means of their radiation. Krypton is named from the Greek word kryptos, “hidden.”
When a current of electricity is passed through a glass tube containing krypton at low pressure, a bluish white light is emitted. The wavelength of an orange-red component of light emitted by stable krypton-86, because of its extreme sharpness, served from 1960 to 1983 as the international standard for the metre. (One metre equaled 1,650,763.73 times the wavelength of this line.)
Compounds
Krypton is the lightest of the noble gases that form isolable chemical compounds in macroscopic amounts. For many years it was considered to be totally unreactive. In the early 1960s, however, krypton was found to react with the element fluorine when both are combined in an electrical-discharge tube at −183 °C (−297 °F); the compound formed is krypton difluoride, KrF2. Several other methods for the synthesis of KrF2 are now known, including irradiation of krypton and fluorine mixtures with ultraviolet radiation at −196 °C (−321 °F).
KrF2 is a colourless crystalline solid that is highly volatile and slowly decomposes at room temperature. No other molecular fluoride of krypton has been isolated, so all krypton compounds are derived from KrF2, where Kr is in the +2 oxidation state. Krypton difluoride is a powerful oxidative fluorinating agent. (Its oxidizing power means that it extracts electrons from other substances and confers on them a positive charge. Its fluorinating ability means that it transfers an F− ion to other substances. Hence, in a formal sense, oxidative fluorination is the net result of extraction of two electrons and addition of F−; this can be considered to be equivalent to the transfer of F+.) KrF2 is, for example, capable of oxidizing and fluorinating xenon to XeF6 and gold to AuF5.
I’m a real element that has a fictional counterpart. What atom am I?
Krypton is an inert (or “noble”) gas and, as such, is not bound up in molecules but exists as single atoms.1 It is the fourth atom in group 18 of the periodic table of elements, after helium, neon, and argon. Its concentration in Earth’s atmosphere is ≈1 ppm by volume.
In 1898, British chemists William Ramsay and Morris Travers discovered krypton as the residue of evaporating almost all of the other components of liquid air. For his work in the discovery of several inert gases, Ramsay was awarded the Nobel Prize in Chemistry in 1904.
Like all inert gases, the boiling and melting points of krypton are only a few degrees apart (see “fast facts”). Krypton has few practical applications, mostly in bright white light bulbs used in photography and in devices used in physical and chemical research. At one time, the wavelength of light emitted by the 86Kr isotope was used to define the meter, but in 1983, the meter’s definition was changed to a function of the speed of light.
In comic books dating back to 1949, “Krypton” was the planet where Superman was born; and “kryptonite” was the only substance that the superhero was vulnerable to. Over the years, the mythological kryptonite was endowed with much more extensive chemistry than real krypton gas.
What is krypton used for
Natural Occurrence
Earth has managed to retain almost all the noble gases except helium. Krypton is naturally present in meteorites and minerals in trace quantities. Krypton concentration in the earth’s atmosphere is around 1 part per million by volume. In other words, for every 100 litres of air, there would be 1/10th of the millilitre of Krypton. An insignificant amount of Krypton could also appear in the earth’s crust due to the decay of uranium and other radioactive elements. The abundance of krypton in space is still uncertain. However, measurements show that all noble gases are abundant in the universe.
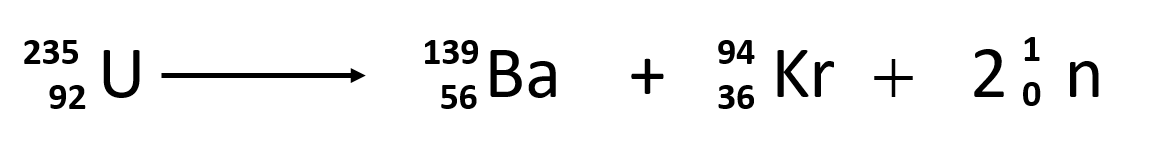
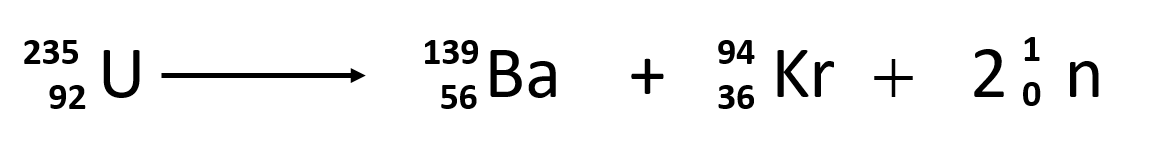
Isotopes
Krypton exists in five stable isotopes and one radioactive isotope (_{ }^{ 78 }{ Kr }78Kr). However, _{ }^{ 78 }{ Kr }78Kr has a very long half-life period of 9200 trillion years, which makes it relatively stable. Krypton-84 is the most prevalent, comprising about 57% of natural krypton. The other five stable isotopes and their relative abundances are krypton-78 (0.4%), krypton-80 (2.3%), krypton-82 (12%), krypton-83 (11%), and krypton-86 (17%). There are thirty other radioactive isotopes and isomers of Krypton out of which only Krypton-81 and Krypton-85 have a half-life long enough to be of any concerns. Other isotopes have a half-life of a maximum of two days.


Properties of Krypton
Krypton is characterised by its brilliant green and orange spectral lines when it is in a gaseous state, whereas solid krypton is white in colour. One should not confuse Krypton with Kryptonite, the Superman repellant. Kryptonite is purely a fictional concept and has nothing to do with Krypton. NO, and there is no real planet by the name Krypton.
Krypton is a colourless (except for solid kryptonite), odourless, and a reasonably expensive element. Like all other inert gases, melting point and boiling point of krypton also have a narrow range. The boiling point of Krypton is –153.4 ºC, and the melting point is –157.4 ºC. The solubility of Krypton in water is about 3 x { 10 }^{ -7 }10−7 mg/L. Krypton is 2.8 times as dense as air at normal temperature and pressure conditions, with a density of 0.003425 gram per cubic centimetre.
Chemical Properties
Krypton has an electronic configuration of [Ar] 3{ d }^{ 10 }4{ s }^{ 2 }4{ p }^{ 6 }3d104s24p6. Like other noble gases, initially, krypton was also considered chemically inert initially. However, after the successful attempt of synthesizing compounds of xenon, scientists were able to isolate a compound Krypton Difluoride KrF2. Under extreme conditions, Krypton reacts with fluorine to form Krypton Difluoride. Krypton, being a member of the noble gas family, usually is unreacted and has an oxidation number of 0. Since the fluoride have an oxidation number of -1 and there are 2 of them in { Kr}{ F}_{ 2 }KrF2, for the compound to be neutral, the Kr must have an oxidation number of +2.