which is true about hydroxide ions
Welcome to solsarin, Keep reading and find the answer about “which is true about hydroxide ions”
which is true of hydroxide ions
Learn about this topic in these articles:
major reference
associated with the presence of hydroxide ions (OH−) in an aqueous solution, and the neutralization of acids by bases could be explained in terms of the reaction of these two ions to give the neutral molecule water (H+ + OH− → H2O). This led naturally to the simple definition that acids…
triphenylphosphine; whereas hard bases include hydroxide, fluoride, and many oxyanions. The dividing line between the hard and soft categories is not a sharp one, and its theoretical interpretation is obscure. Nevertheless, a surprising amount of factual information can be coordinated on the basis of preferential reactions of hard acids.
A simple definition for acid is “any substance that, when dissolved in water, produces hydronium ions (H3O+)”. Hydronium ions are produced when an H+ ion from a proton donor reacts with water. For example, when hydrochloric acid is dissolved in water the following reaction occurs:
H3O+(aq) + Cl–(aq)
Bases can be defined as “any substance that, when dissolved in water, generates hydroxide ions (OH–)”. Hydroxide ions are produced under two conditions. First, they are produced when an ionic compound containing hydroxide such as sodium hydroxide dissolves in water.
Second, hydroxide ions are produced when a substance like ammonia accepts a proton from water.
In all aqueous (water) solutions, the product of the hydronium ion and hydroxide ion concentrations is constant. If the concentrations are expressed in molarity (indicated by square brackets) the constant is called Kw and is equal to 1 x 10-14 at 25oC. The formula appears below.
In pure water and in neutral solutions, the [H3O+] and [OH–] are equal. Therefore, [H3O+] = [OH–] = 1 x10-7.
However, this can be altered by the addition of an acid or base. If an acid is added to pure water the concentration of hydronium ions in the solution increases. In order for [H3O+] [OH–] to remain constant, the hydroxide ion concentration must decrease. As a result [H3O+] > [OH–] and the solution is said to be acidic. If a base is added to pure water, the reverse is true. When the concentration of hydroxide ions in a solution increases the hydronium ion concentration must decrease. In this case [H3O+] < [OH–] and the solution is considered basic.
Continue to the next background page and read about pH.
Hydrogen and Hydroxide Ions
If you refer to Table 1 from Ions in Solution (Electrolytes), you will see that pure water does conduct some electrical current, albeit much less than even the weak electrolytes listed there. This is because water itself is a very weak electrolyte. It ionizes to hydrogen ions and hydroxide ions to an extremely small extent:
Careful measurements show that at 25°C the concentrations of H+(aq) and OH–(aq) are each 1.005 × 107 mol dm–3. At higher temperatures more H+(aq) and OH–(aq) are produced while at lower temperatures less ionization of water occurs. Nevertheless, in pure water, the concentration of H+(aq) always equals the concentration of OH–(aq). Dissolving acids or bases in water can change the concentrations of both H+(aq) and OH–(aq), causing them to differ from one another. The special case of a solution in which these two concentrations remain equal is called a neutral solution.
A hydrogen ion, H+, is a hydrogen atom that has lost its single electron; that is, a hydrogen ion is just a proton. Because a proton is only about one ten-thousandth as big as an average atom or ion, water dipoles can approach very close to a hydrogen ion in solution. Consequently, the proton can exert a very strong attractive force on a lone pair of electrons in a water molecule—strong enough to form a coordinate covalent bond:


The H3O+ formed in this way is called a hydronium ion (on the left in the figure below). All three of its O―H bonds are exactly the same, and the ion has a pyramidal structure as predicted by VSEPR theory (1a). To emphasize the fact that a proton cannot exist by itself in an aqueous solution, Eq. (1a) is often rewritten as


Like other ions in an aqueous solution, both hydronium and hydroxide ions are hydrated. Moreover, hydrogen bonds are involved in attracting water molecules to hydronium and hydroxide ions. In both cases three water molecules appear to be rather tightly held, giving formulas H3O(H2O)3+ (or H9O4+) and HO(H2O)3– (or H7O4–). Possible structures for the hydrated hydronium and hydroxide ions are shown in Figure 11.5.1.
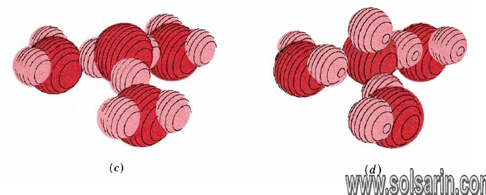

Hydrogen bonding of hydronium and hydroxide ions to water molecules accounts rather nicely for the unusually large electrical currents observed for some electrolytes containing H and OH. The case of the hydronium ion is illustrated in Figure 11.5.2. When a hydronium ion collides with one end of a hydrogen-bonded chain of water molecules, a different hydronium ion can be released at the other end.
Only a slight movement of six protons and a rearrangement of covalent and hydrogen bonds are needed. In effect, a hydronium ion can almost instantaneously “jump” the length of several water molecules. It need not elbow its way through a crowd as other ions must. The same is true of aqueous hydroxide ions. Since both ions move faster, they can transfer more electrical charge per unit time, that is, more current.
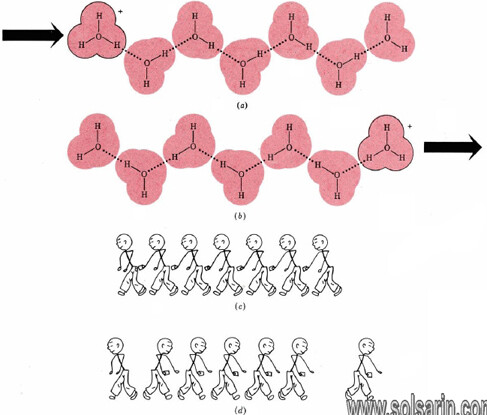

Figure 11.5.2 Rapid transfer of protons through the aqueous solution. (a) Hydronium ion is hydrogen-bonded to a chain of water molecules. Minor rearrangement of the positions of H nuclei and hydrogen bonds results in the situation (b) where the hydronium ion is at the right end of the chain. (c) An analogous situation would be a row of pickpockets in a crowd. The net result is the transfer of a wallet from the person at the left of the row to the right (d). Only the ends of the chain have gained or lost wallets.
Read More Post:
Applications
Sodium hydroxide solutions, also known as lye and caustic soda, are used in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents, and as a drain cleaner. Worldwide production in 2004 was approximately 60 million tonnes. The principal method of manufacture is the chloralkali process.
Solutions containing the hydroxide ion are generated when a salt of a weak acid is dissolved in water. Sodium carbonate is used as an alkali, for example, by virtue of the hydrolysis reaction
- CO2−
3 + H2O ⇌ HCO−
3 + OH− (pKa2= 10.33 at 25 °C and zero ionic strength)
Although the base strength of sodium carbonate solutions is lower than a concentrated sodium hydroxide solution, it has the advantage of being solid. It is also manufactured on a vast scale (42 million tonnes in 2005) by the Solvay process. An example of the use of sodium carbonate as an alkali is when washing soda (another name for sodium carbonate) acts on insoluble esters, such as triglycerides, commonly known as fats, to hydrolyze them and make them soluble.
Bauxite, a basic hydroxide of aluminum, is the principal ore from which the metal is manufactured. Similarly, goethite (α-FeO(OH)) and lepidocrocite (γ-FeO(OH)), basic hydroxides of iron, are among the principal ores used for the manufacture of metallic iron.
Carbon group elements
Carbon forms no simple hydroxides. The hypothetical compound C(OH)4 (orthocarbonic acid or methanetetrol) is unstable in an aqueous solution:
- C(OH)4 → HCO−
3 + H3O+ - HCO−
3 + H+ ⇌ H2CO3
Carbon dioxide is also known as carbonic anhydride, meaning that it forms by dehydration of carbonic acid H2CO3 (OC(OH)2).
Silicic acid is the name given to a variety of compounds with a generic formula [SiOx(OH)4−2x]n. Orthosilicic acid has been identified in very dilute aqueous solution. It is a weak acid with pKa1 = 9.84, pKa2 = 13.2 at 25 °C. It is usually written as H4SiO4, but the formula Si(OH)4 is generally accepted.[dubious – discuss] Other silicic acids such as metasilicic acid (H2SiO3), silicic acid (H2Si2O5), and pyrosilicic acid (H6Si2O7) have been characterized. These acids also have hydroxide groups attached to the silicon; the formulas suggest that these acids are protonated forms of poly oxyanions.
Few hydroxo complexes of germanium have been characterized. Tin(II) hydroxide Sn(OH)2 was prepared in anhydrous media. When tin(II) oxide is treated with alkali the pyramidal hydroxo complex Sn(OH)−.
3 is formed. When solutions containing this ion are acidified, the ion [Sn3(OH)4]2+ is formed together with some basic hydroxo complexes. The structure of [Sn3(OH)4]2+ has a triangle of tin atoms connected by bridging hydroxide groups. Tin(IV) hydroxide is unknown but can be regarded as the hypothetical acid from which stannates, with a formula [Sn(OH)6]2−, are derived by reaction with the (Lewis) basic hydroxide ion.
Hydrolysis of Pb2+ in an aqueous solution is accompanied by the formation of various hydroxo-containing complexes, some of which are insoluble. The basic hydroxo complex [Pb6O(OH)6]4+ is a cluster of six lead centers with metal-metal bonds surrounding a central oxide ion. The six hydroxide groups lie on the faces of the two external Pb4 tetrahedra. In strongly alkaline solutions soluble plumbate ions are formed, including [Pb(OH)6]2−



