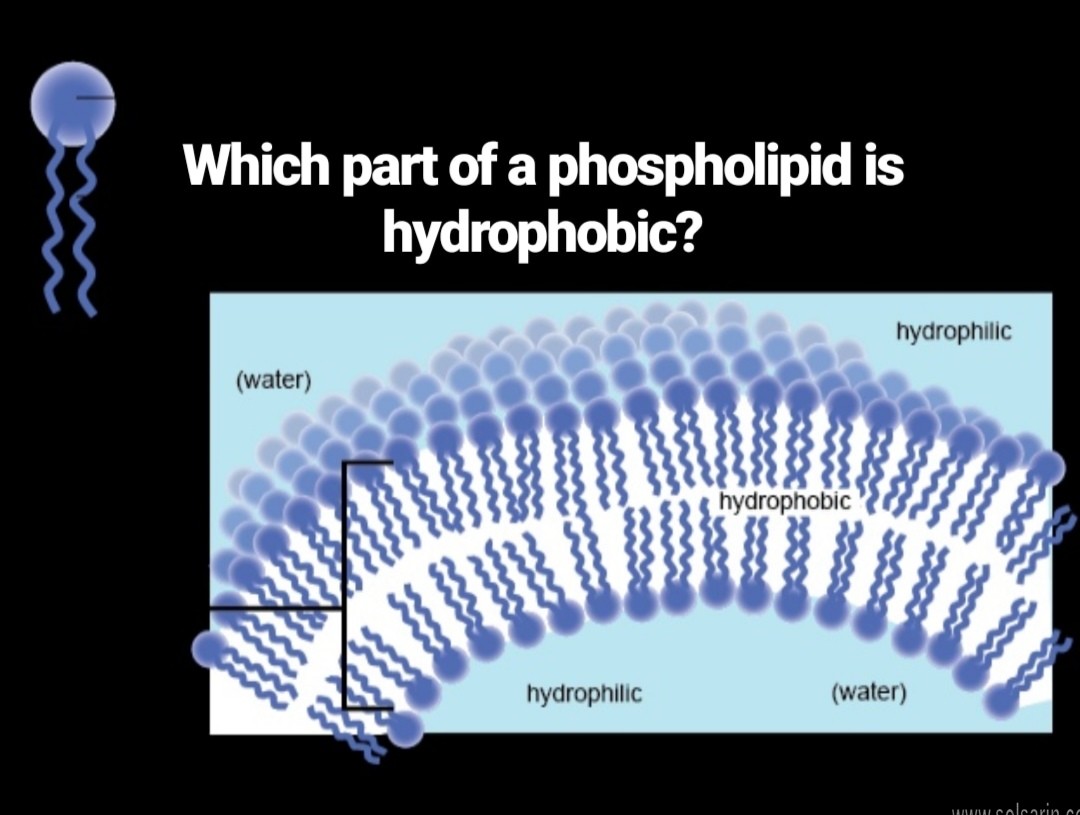
which part of a phospholipid is hydrophobic?
Hi, welcome to solsarin site, in this post we want to talk about“which part of a phospholipid is hydrophobic”,
thank you for choosing us.
which part of a phospholipid is hydrophobic?
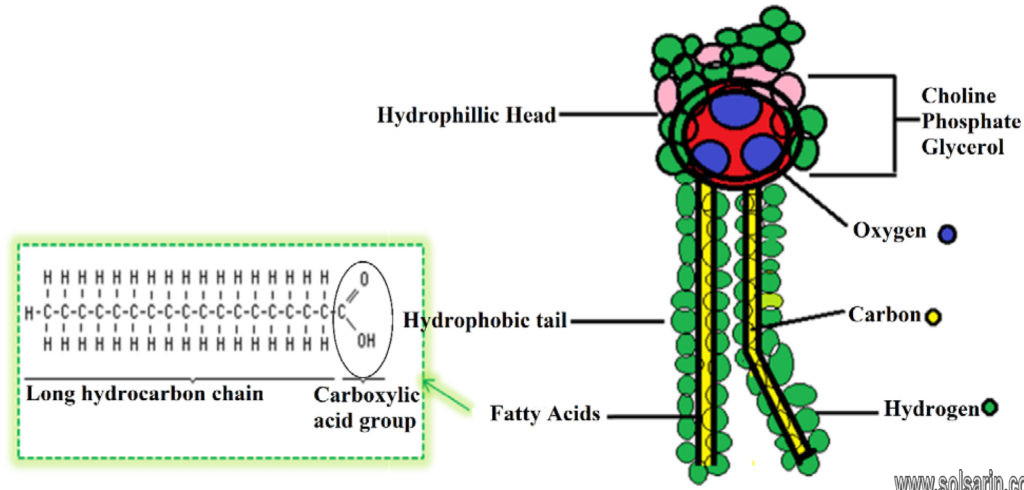
Phospholipids consist of a glycerol molecule, two fatty acids, and a phosphate group that is modified by an alcohol. The phosphate group is the negatively-charged polar head, which is hydrophilic. The fatty acid chains are the uncharged, nonpolar tails, which are hydrophobic.
Are phospholipids hydrophobic?
Phospholipids are amphiphilic molecules with hydrophobic fatty acid chains and hydrophilic moieties. They occur naturally in all living organisms as the major components of cell membranes.
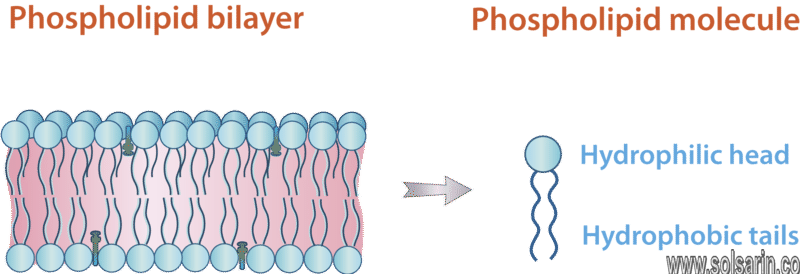

Defining Characteristics of Phospholipids
Phospholipids are major components of the plasma membrane, the outermost layer of animal cells. Like fats, they are composed of fatty acid chains attached to a glycerol backbone. Unlike triglycerides, which have three fatty acids, phospholipids have two fatty acids that help form a diacylglycerol.
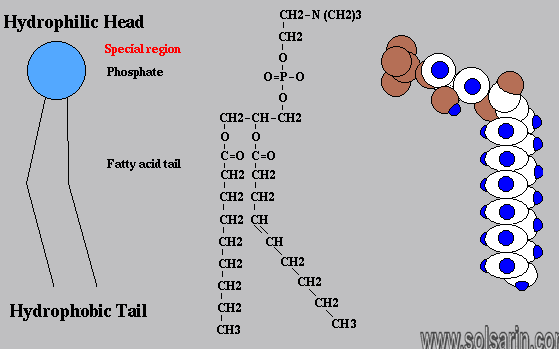
The third carbon of the glycerol backbone is also occupied by a modified phosphate group However, just a phosphate group attached to a diacylglycerol does not qualify as a phospholipid. This would be considered a phosphatidate (diacylglycerol 3-phosphate), the precursor to phospholipids. To qualify as a phospholipid, the phosphate group should be modified by an alcohol. Phosphatidylcholine and phosphatidylserine are examples of two important phospholipids that are found in plasma membranes.
Phospholipid MoleculeA phospholipid is a molecule with two fatty acids and a modified phosphate group attached to a glycerol backbone. The phosphate may be modified by the addition of charged or polar chemical groups. Two chemical groups that may modify the phosphate, choline and serine, are shown here. Both choline and serine attach to the phosphate group at the position labeled R via the hydroxyl group indicated in green.
Phospholipids

Functions of Phospholipids
As membrane components, phospholipids are selectively permeable (also called semi-permeable), meaning that only certain molecules can pass through them to enter or exit the cell. Molecules that dissolve in fat can pass through easily, while molecules that dissolve in water cannot. Oxygen, carbon dioxide, and urea are some molecules that can pass through the cell membrane easily. Large molecules like glucose or ions like sodium and potassium cannot pass through easily. This helps keep the contents of the cell working properly and separates the inside of the cell from the surrounding environment.
Phospholipids can be broken down in the cell and used for energy. They can also be split into smaller molecules called chemokines, which regulate a variety of activities in the cell such as production of certain proteins and migration of cells to different areas of the body. Additionally, they are found in areas such as the lung and in joints, where they help lubricate cells.
In pharmaceuticals, phospholipids are used as part of drug delivery systems, which are systems that help transport a drug throughout the body to the area that it is meant to affect. They have high bioavailability, meaning that they are easy for the body to absorb. Valium is an example of a medication that uses a phospholipid-based drug delivery system.
In the food industry, phospholipids can act as emulsifiers, which are substances that disperse oil droplets in water so that the oil and water do not form separate layers. For example, egg yolks contain phospholipids, and are used in mayonnaise to keep it from separating. Phospholipids are found in high concentrations in many other animal and plant sources, such as soybeans, sunflowers, cotton seeds, corn, and even cow brains.
Phospholipid bilayer
Once the bilayer forms, further stabilization comes from other weak forces. Van der Waals forces can result from the packing of fatty acid tails inside the bilayer. Hydrogen bonding and electrostatic attractions (ionic bonds) occur between the hydrophilic groups of phospholipids and the aqueous solution.
We say that hydrophobic forces cause the bilayer to form, and the other weak forces stabilize the bilayer. This example of one weak force initiating a structural rearrangement that is then stabilized by other weak forces is repeated many times in biology.
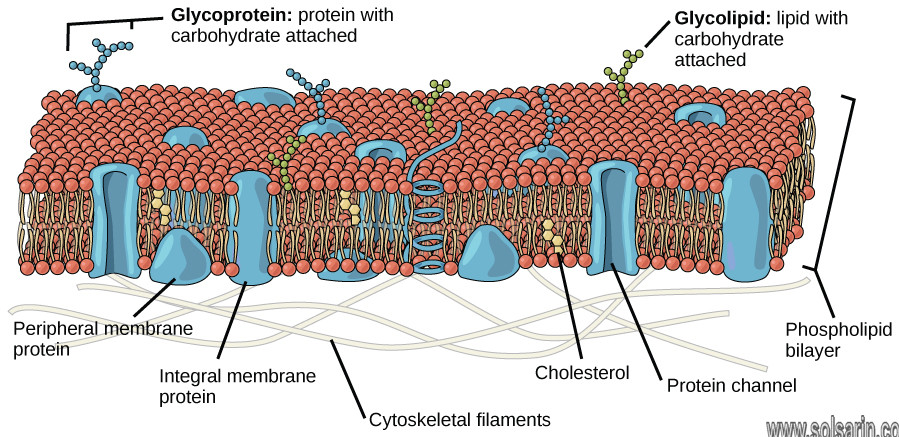

Properties of the Phospholipid Bilayer:
- The bilayer is held together by weak hydrophobic interactions between the tails
- Hydrophilic / hydrophobic layers restrict the passage of many substances
- Individual phospholipids can move within the bilayer, allowing for membrane fluidity and flexibility
- This fluidity allows for the spontaneous breaking and reforming of membranes (endocytosis / exocytosis)
The cell membrane is composed mainly of phospholipids, which consist of fatty acids and alcohol. The phospholipids in the cell membrane are arranged in two layers, called a phospholipid bilayer. Each phospholipid molecule has a head and two tails. The head “loves” water (hydrophilic) and the tails “hate” water (hydrophobic). The water-hating tails are on the interior of the membrane, whereas the water-loving heads point outwards, toward either the cytoplasm or the fluid that surrounds the cell.
Molecules that are hydrophobic can easily pass through the cell membrane, if they are small enough, because they are water-hating like the interior of the membrane. Small lipids and steroids are hydrophobic and can readily cross the membrane. Molecules that are hydrophilic, on the other hand, cannot pass through the cell membrane—at least not without help—because they are water-loving like the exterior of the membrane, and are therefore excluded from the interior of the membrane. Hydrophilic molecules such as glucose and ions like Na+ and K+ need the help of special proteins to cross the membrane.
Classification of phospholipids
Depending on the structure of this alcohol, different types of phospholipids comprise, for example, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), or phosphatidylserine (PS) The specific and non-random distribution of substituents over the positions sn-1, sn-2, and sn-3 of the glycerol introduces chirality. Depending on the structure of the polar headgroup and pH of the surrounding medium, PE and PC are zwitterionic and have a neutral charge at pH 7, whereas PG, PI, and PS are negatively charged at this pH value.
What forms the tail of phospholipid?

Nomenclature of phospholipids
The phospholipid may have two esterified fatty acids and are called diacyl-phospholipids,
whereas phospholipids with one fatty acid are called monoacylphospholipids or lyso-phospholipids.
In scientific literature, synthetic phospholipids are named in an abbreviated way according to
“number of the position of fatty acids—type of fatty acid—phosphatidyl—alcohol”. The number of fatty acids could be ‘mono’ or ‘di’, the fatty acids are described as, e.g., oleoyl or palmitoyl (coming from oleic acid and palmitic acid, respectively), ‘phosphatidyl’ describes the backbone of the
phospholipid molecule which encompasses glycerol, further esterified with one or two fatty acid and a phosphate group, and finally the alcohol is mentioned as described above. The International Union of Pure and Applied Chemistry (IUPAC) instead uses “glycero—phospho—alcohol”. Examples are POPC, 1-palmitoyl-2-oleoylphosphatidylcholine (IUPAC: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) or DOPE, 1,2-dioleoylphosphatidylethanolamine (IUPAC: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine).
Since the number, type and position of the fatty acid as well as the type of headgroup can be varied
modularly, there is a very large number of different phospholipids in nature. Depending on the origin of the natural phospholipids (plant or animal sources), the composition of fatty acids differs.