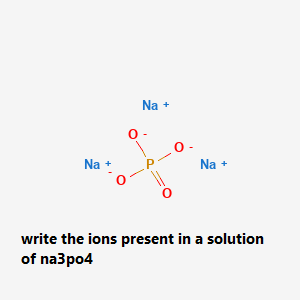
write the ions present in a solution of na3po4
Hello dear readers. In this post on Solsarin we are going to talk about ”write the ions present in a solution of na3po4“ . Continue reading to find the answer. Please write your comment, Thanks for your attention.
Write the ions present in a solution of na3po4:
The ions present in the solution of Na₃PO₄ are:
3Na⁺¹ and 1PO₄⁻³
there are 3 sodium ions (Na⁺¹) are present, these are cations (+).
And 1 phosphate ion (PO₄⁻³) is present, this is anion (-),
When these cations and ions meet together a compound is formed, in this case 3 sodium ions make a bond with 3 oxygens of phosphate and makes a compound of sodium phosphate.


Ions
Ions are atoms or groups of atoms that have a charge. A cation has lost electrons, so it has a positive charge. An anion has gained electrons, so it has a negative charge. Polyatomic ions contain more than one atom.
The ions present in Na3 PO4 are Na+ and PO4 3-. Sodium is found in the alkali metals on the periodic table, which indicates it will form a +1 charge in solution. The phosphate ion is an example of a polyatomic ion, whose charge must be memorized.
What ions are present in a solution of Na3PO4?
In a redox reaction, a spectator ion is an ion whose oxidation number does not change in the course of the reaction. For example, consider the redox reaction:
2Al+3CuCl2→2AlCl3+3Cu2Al+3CuCl2→2AlCl3+3Cu
In this reaction, the chloride ion is a spectator ion since its oxidation number doesn’t change. In order to know that, though, you have to assign oxidation numbers to each of the elements involved in the reaction.
You can rewrite the above equation without the chloride ion:
2Al+3Cu2+→2Al3++3Cu2Al+3Cu2+→2Al3++3Cu
In a double replacement reaction, there are no oxidation number changes. In these situations, spectator ions are ions that do not combine to form insoluble precipitates. Consider the following double replacement reaction:
AgNO3(aq)+NaCl(aq)→AgCl(s)+NaNO3(aq)AgNO3(aq)+NaCl(aq)→AgCl(s)+NaNO3(aq)
When an ionic compound is dissolved in water, its ions dissociate, so you could write the equation like this:
Ag+(aq)+NO−3(aq)+Na+(aq)+Cl−(aq)→AgCl(s)+Na+(aq)+NO−3(aq)Ag+(aq)+NO3−(aq)+Na+(aq)+Cl−(aq)→AgCl(s)+Na+(aq)+NO3−(aq)
Now you’ll notice that the sodium (Na+Na+) and nitrate (NO−3NO3−) ions are present on both sides of the equation, which means they can be removed from the equation. The sodium and nitrate ions are spectator ions in this case — nothing happens to them from one side of the equation to the other. Here’s the net ionic equation:
Ag+(aq)+Cl−(aq)→AgCl(s)
How many ions are in na3po4?
there are 3 sodium ions (Na⁺¹) are present, these are cations (+). And 1 phosphate ion (PO₄⁻³) is present, this is anion (-), When these cations and ions meet together a compound is formed, in this case 3 sodium ions make a bond with 3 oxygens of phosphate and makes a compound of sodium phosphate.
What is the charge of sodium phosphate?
Sodium Phosphate is an ionic compound formed by two ions, Sodium Na+ and Phosphate PO−34 . In order for these two polyatomic ions to bond the charges must be equal and opposite. Therefore, it will take three +1 sodium ions to balance the one -3 phosphate ion.
Do sodium ions have a 1+ charge?
Forming positive ions A sodium atom has one electron in its outer shell. It will still have 11 positive protons but only 10 negative electrons. So, the overall charge is +1. A positive sign is added to the symbol for sodium, Na +.
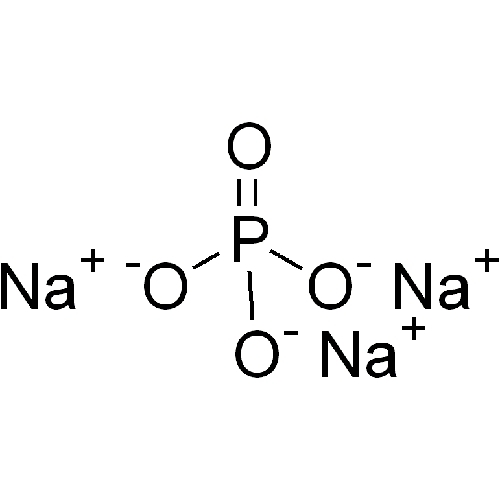
What ions are present in na3po4?
Answer and Explanation: The ions present in Na3 PO4 are Na+ and PO4 3-. Sodium is found in the alkali metals on the periodic table, which indicates it will form a +1 charge in solution. The phosphate ion is an example of a polyatomic ion, whose charge must be memorized.
What is the formula for sodium phosphate?
Na₃PO₄
What ions are present in PbCl4?
a) PbCl4 is bonded covalently with a single shared pair of electrons between each chlorine and the lead atom. PbCl2 can be thought of as ionic and is held together by attractions between Pb2+ ions and Cl- ions.
What PbCl4 called?
Lead tetrachloride | PbCl4 – PubChem.
How many ions are present in Al clo4 3?
Answer Expert Verified. Aluminum chlorate ionizes as follows, 1 mol Aluminum chlorate ionizes to give 1 mole Aluminum ion and 3 moles of chlorate ion.
Is Al ClO4 3 an acid or base?
So when comparing say 1 M of KBr, Al(ClO4)3, CsF, and AlF3; KBr is neutral because KOH is a strong base and HBr is a strong acid, Al(ClO4)3 is acidic because ClO4 is part of a strong acid, CsF is basic because Cs is part of a strong base…
What is the name of al ClO4 3?
Aluminum Perchlorate Al
What is the molar mass of Al ClO4 3?
325.3333
What is Zn hco32?
zinc bicarbonate
What is the name of K3AsO4?
Potassium Arsenate
What is the chemical formula for Aluminium?
Aluminum can be quantitatively analyzed as the oxide (formula Al2O3) or as a derivative of the organic nitrogen compound 8-hydroxyquinoline. The derivative has the molecular formula Al(C9H6ON)3.
Is Aluminium a good conductor of electricity?
Copper and aluminium are most frequently used as the electrical conductors in electrical cables due to their low resistance and excellent conductivity. These metals are both ductile and relatively resistant to corrosion, but they also have different properties which make them useful for various applications.
How do you calculate aluminum?
Aluminum has an atomic weight of 26.9815 in Daltons or grams per mole (g/mol). This means if you have a certain number of moles of aluminum, you can multiply it by the atomic weight to figure out how many grams per mole of aluminum you have. This accounts for the weight of aluminum in its pure form.


What do we use aluminum for?
Aluminium is a silvery-white, lightweight metal. It is soft and malleable. Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts.
What are 5 uses of Aluminium?
Below are ten of the most common and useful applications of aluminium in modern society.
- Power lines.
- High-rise buildings.
- Window frames.
- Consumer electronics.
- Household and industrial appliances.
- Aircraft components.
- Spacecraft components.
- Ships.
What industry uses the most aluminum?
automobile industry

MORE POSTS FOR READERS:
- it goes through central africa
- Michigan State Trunkline Highway System
- rubik’s cube
- what is pandora box movie about
- can kiwis fly?
What are three interesting facts about aluminum?
7 Fast Facts About Aluminum
- #1) It Weighs One-Third Less Than Steel.
- #2) It Doesn’t Rust.
- #3) It’s the World’s Most Abundant Metal.
- #4) It’s Recyclable.
- #5) It Was Used Thousands of Years Ago.
- #6) It’s Resistant to Heat.
- #7) It’s Ductile.
Is aluminum toxic to the body?
Human exposure to aluminium is inevitable and, perhaps, inestimable. Aluminium’s free metal cation, Alaq(3+), is highly biologically reactive and biologically available aluminium is non-essential and essentially toxic.