A disaccharide forms when
Hey guys! We return with an amazing topic about science in solsarin. This is “A disaccharide forms when” which is really interesting. I suggest you to stay along with us and tell us your comments.

Disaccharide
A disaccharide (also called a double sugar or biose) is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose.
Disaccharides are one of the four chemical groupings of carbohydrates (monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule.
The joining of monosaccharides into a double sugar happens by a condensation reaction, which involves the elimination of a water molecule from the functional groups only. Breaking apart a double sugar into its two monosaccharides is accomplished by hydrolysis with the help of a type of enzyme called a disaccharidase. As building the larger sugar ejects a water molecule, breaking it down consumes a water molecule. These reactions are vital in metabolism. Each disaccharide is broken down with the help of a corresponding disaccharidase (sucrase, lactase, and maltase).
Classification
There are two functionally different classes of disaccharides:
- Reducing disaccharides, in which one monosaccharide, the reducing sugar of the pair, still has a free hemiacetal unit that can perform as a reducing aldehyde group; lactose, maltose and cellobiose are examples of reducing disaccharides, each with one hemiacetal unit, the other occupied by the glycosidic bond, which prevents it from acting as a reducing agent. They can easily be detected by the Woehlk test or Fearon’s test on methylamine.
- Non-reducing disaccharides, in which the component monosaccharides bond through an acetal linkage between their anomeric centers. This results in neither monosaccharide being left with a hemiacetal unit that is free to act as a reducing agent. Sucrose and trehalose are examples of non-reducing disaccharides because their glycosidic bond is between their respective hemiacetal carbon atoms. The reduced chemical reactivity of the non-reducing sugars in comparison to reducing sugars, may be an advantage where stability in storage is important.
Formation
The formation of a disaccharide molecule from two monosaccharide molecules proceeds by displacing a hydroxy group from one molecule and a hydrogen nucleus (a proton) from the other, so that the now vacant bonds on the monosaccharides join the two monomers together. Because of the removal of the water molecule from the product, the term of convenience for such a process is “dehydration reaction” (also “condensation reaction” or “dehydration synthesis”).
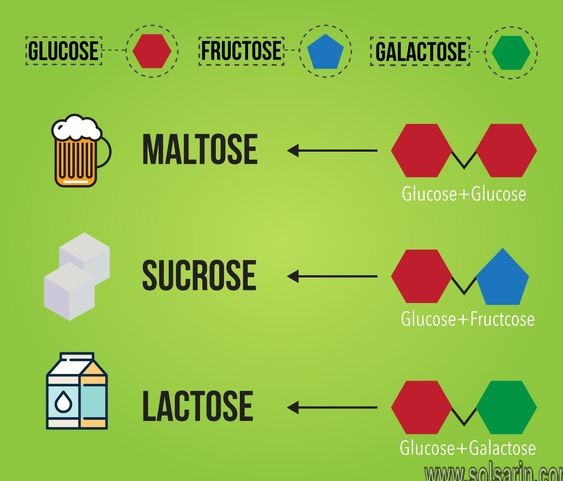
For example, milk sugar (lactose) is a disaccharide made by condensation of one molecule of each of the monosaccharides glucose and galactose, whereas the disaccharide sucrose in sugar cane and sugar beet, is a condensation product of glucose and fructose. Maltose, another common disaccharide, is condensed from two glucose molecules.
The dehydration reaction that bonds monosaccharides into disaccharides (and also bonds monosaccharides into more complex polysaccharides) forms what are called glycosidic bonds.
Bonds and Properties
Note different disaccharides are conceivable when monosaccharides attach to one another since a glycosidic bond can shape between any hydroxyl total on the segment sugars. For instance, two glucose atoms can join to shape maltose, trehalose, or cellobiose.
Despite the fact that these disaccharides are produced using similar segment sugar, they are unmistakable particles with various substances and physical properties from one another.
At the point when the anorexic hydroxyl gathering of one monosaccharide is bound glycosidically with one of the OH gatherings of another, a disaccharide is framed. As in all glycosidic, the glycosidic bond does not let mutarotation. Since this kind of bond is shaped stereospecifically by catalysts in characteristic disaccharides, they are just found in one of the conceivable setups (α or β).

Uses of Disaccharides
Monosaccharides are utilized as energy bearers and to proficiently transport disaccharides. Explicit instances of uses include:
In the human body and indifferent creatures, sucrose is processed and broken into its segment basic sugars for speedy energy. Abundance sucrose can be changed over from a cube of sugar into a lipid for capacity as fat. Sucrose has a sweet flavor.
Lactose (milk sugar) is found in human bosom milk, where it fills in as a concoction energy hotspot for newborn children.
Lactose, like sucrose, has a sweet flavor. As people age, lactose turns out to be less-endured. This is on the grounds that lactose assimilation requires the enzyme lactase. Individuals who are lactose prejudiced can take a lactase supplement to decrease swelling, cramping, sickness, and runs.
Plants use disaccharides to transport fructose, glucose, and galactose starting with one cell then onto the next.
Maltose, in contrast to some different disaccharides, does not fill a need in the human body. The sugar liquor type of maltose is maltitol, which is utilized in sans sugar nourishments. Obviously, maltose is sugar, yet it is not completely processed and consumed by the body (50 to 60 percent).
Types of Disaccharides
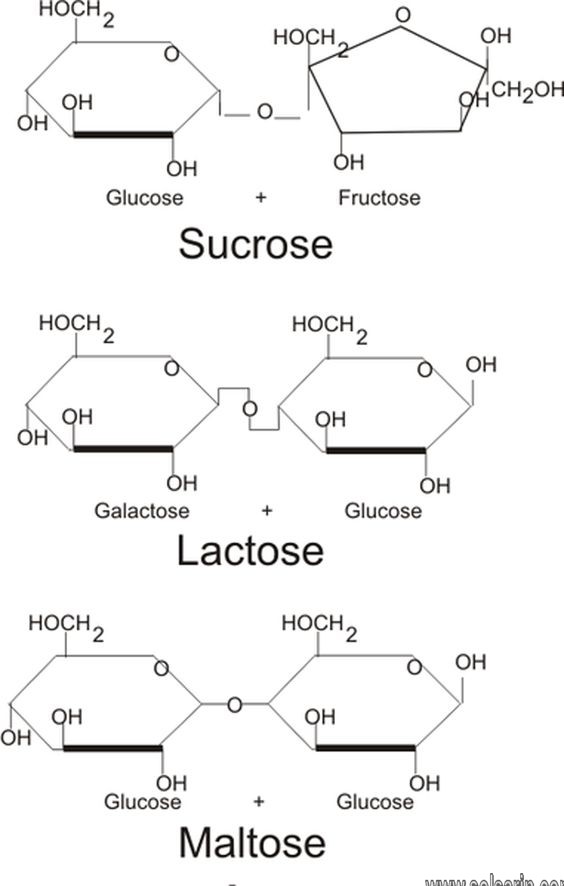
Sucrose
This is the most important disaccharide. It is popularly known as table sugar. Sucrose is found in all photosynthetic plants. It is commercially obtained from sugarcane and sugar beets via an industrial process. Let us take a look at some chemical properties of sucrose
- The molecular formula of sucrose is C12H22O11.
- If sucrose goes through acid catalysed hydrolysis it will give one mole of D-Glucose and one mole of D-Fructose.
- The chemical structure of sucrose comprises of α form of glucose and β form of fructose
- The glycosidic linkage is α linkage because the molecule formation is in α orientation
- Sucrose is a non-reducing sugar. As you can see from the structure it is combined (linked) at the hemiacetal oxygen and does not have a free hemiacetal hydroxide
- Since has no free hemiacetal hydroxide it does not show mutarotation (α to β conversion). Sucrose also does not form osazones for the same reason.
- We can prove the structural formula of sucrose by hydrolysing it with α-glycosidase enzymes which only hydrolyses α glucose. This test is positive for sucrose.
Lactose
This is a disaccharide you may already be familiar with. Lactose is the primary ingredient found in the milk of all mammals. Unlike the majority of saccharides, lactose is not sweet to taste. Lactose consists of one galactose carbohydrate and one glucose carbohydrate. These are bound together by a 1-4 glycosidic bond in a beta orientation.
If you look at the structure of lactose you will see that there is one significant difference between galactose and glucose. Galactose’s fourth carbon has a different orientation in galactose than in sucrose. If it was not so the resulting molecule would have just been sucrose (glucose+glucose) instead of lactose.
Also from the structure, we can notice that lactose is a reacting sugar since it has one free hemiacetal hydroxide. So when we react Lactose with bromine water it will give monocarboxylic acid.
Maltose
Maltose is another disaccharide commonly found. It has two monosaccharide glucose molecules bound together, The link is between the first carbon atom of glucose and the fourth carbon of another glucose molecule. This, as you know, is the one-four glycosidic linkage. Let us look at a few of its properties
- On acid catalysed hydrolysis one mole of maltose gives two moles of D-glucose.
- Maltose has a free hemiacetal hydroxide, hence it undergoes mutarotation. It exists as both α-Maltose and also β-Maltose
- For the same reasons it also gives a positive test with Benedicts and Tollens reagent.
Some More Types of Disaccharides
There few more types which are not that popular, such as:
-
Trehalose
It is made up of 2 molecules of glucose which are linked differently. This can be found in fungi, plants, and insects.
-
Lactulose
It is formed from galactose and fructose. It is helpful for the treatment of constipation and liver diseases.
-
Cellobiose
It is also made up of two glucose molecules which are also arranged differently. These can be seen bacteriology which is a form of chemical analysis.
-
Chitobiose
It comprises two glucosamine molecules which are linked. It is seen in some bacteria, exoskeletons of insects and is also found in fish, octopus, and squid.
For more information on topics like glucose and more, register with BYJU’S and download our app.

Summary
Maltose is composed of two molecules of glucose joined by an α-1,4-glycosidic linkage. It is a reducing sugar that is found in sprouting grain. Lactose is composed of a molecule of galactose joined to a molecule of glucose by a β-1,4-glycosidic linkage. It is a reducing sugar that is found in milk. Sucrose is composed of a molecule of glucose joined to a molecule of fructose by an α-1,β-2-glycosidic linkage. It is a nonreducing sugar that is found in sugar cane and sugar beets.