how to calculate percentage of water in a hydrate
Hello welcome to the solsarin site. stay with us to see ” how to calculate percentage of water in a hydrate ”
CONCEPT:
A HYDRATE is a compound that incorporates water molecules into its fundamental solid structure. In a hydrate (which usually has a specific crystalline form), a defined number of water molecules are associated with each formula unit of the primary material.
Gypsum is a hydrate in which two water molecules are present for every formula unit of CaSO4 in the solid. The chemical formula for gypsum is CaSO4 ·2 H2O and the chemical name is calcium sulfate dihydrate. Note that the dot in the formula (or multiplication sign) indicates that the waters are there.
Other examples of hydrates are: lithium perchlorate trihydrate – LiClO4 ·3H2O; magnesium carbonate pentahydrate – MgCO3 ·5 H2O; copper(II) sulfate pentahydrate – CuSO4 ·5 H2O; Nickel(II) sulfate hexahydrate – NiSO4 ·6 H2O; and aluminum potassium sulfate dodecahydrate – AlK(SO4)2 ·12 H2O
This water in the hydrate (referred to as “water of hydration”) can be removed by heating the hydrate. When all hydrating water is removed, the material is said to be anhydrous and is referred to as an anhydrate.
CuSO4 ·5 H2O(s) + HEAT —> CuSO4 (s) + 5 H2O (g)
Hydrate Anhydrate
EXPERIMENTAL MEASUREMENT OF PERCENT HYDRATION:
Experimentally measuring the percent water in a hydrate involves first heating a known mass of the hydrate to remove the waters of hydration and then measuring the mass of the anhydrate remaining. The difference between the two masses is the mass of water lost. Dividing the mass of the water lost by the original mass of hydrate used is equal to the fraction of water in the compound. Multiplying this fraction by 100 gives the percent water in the hydrate.
EXAMPLE 1:
When a 1.000 g sample of CuSO4 ·5 H2O(s) was heated so that the waters of hydration were driven off, the mass of the anhydrous salt remaining was found to be 0.6390 g. What is the experimental value of the percent water of hydration?
CuSO4 ·5 H2O(s) + HEAT —> CuSO4 (s) + 5 H2O (g)
1.000 g 0.6390 g
1. Find the difference between the mass of hydrate before heating and the mass of the anhydrate after heating. The difference is the mass of water last.
1.000 g – 0.6390 g = 0.3610 g
2. Dividing the mass of the water lost by the mass of hydrate used is equal to the fraction of water in the compound. Multiplying this fraction by 100 gives the percent water in the hydrate.
(0.3610 g /1.000 g)(100) = 36.10%
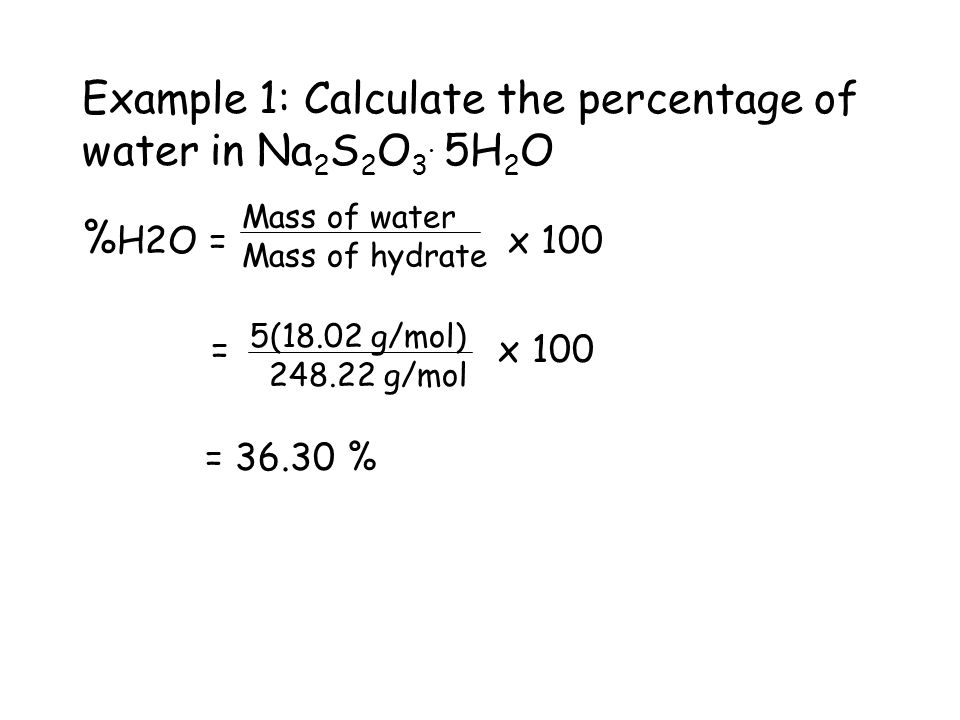
CALCULATION OF PERCENT HYDRATION FROM THE CHEMICAL FORMULA
The theoretical (actual) percent hydration (percent water) can be calculated from the formula of the hydrate by dividing the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiplying this fraction by 100.
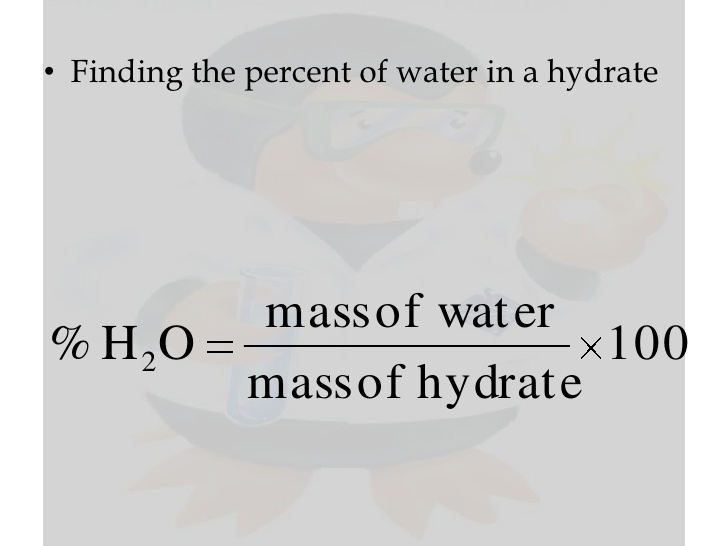
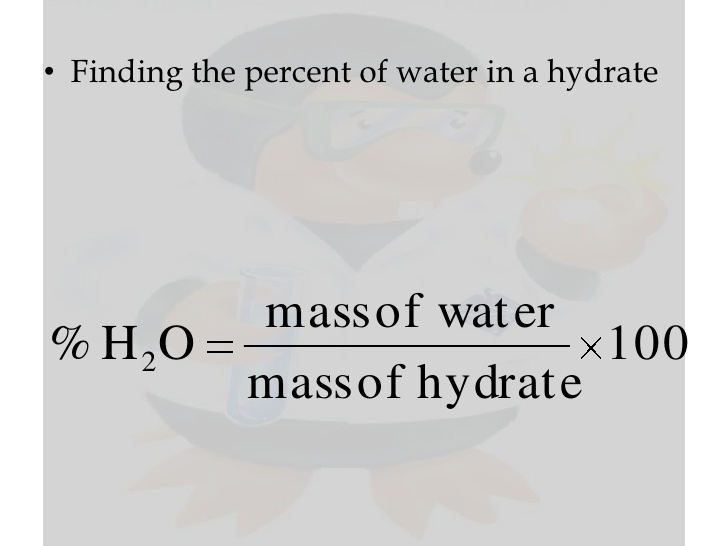
EXAMPLE 2:
What is the percent water in copper(II) sulfate pentahydrate, CuSO4 ·5 H2O?
1. Calculate the formula mass. When determining the formula mass for a hydrate, the waters of hydration must be included.
1 Cu @ 63.55 g = 63.55 g
1 S @ 32.07 g = 32.07 g 2 H @ 1.01 = 2.02 g
4 O @ 16.00 g = 64.00 g 1 O @ 16.00 = 16.00 g
159.62 g/mol 18.02 g/mol
Formula Mass = 159.62 + 5(18.02) = 249.72 g/mol
2. Divide the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiply this fraction by 100.
Percent hydration = (90.10 g /249.72 g)(100) = 36.08%
CALCULATION OF THE PERCENT ERROR
Percent error = ( theoretical – experimental / theoretical value) x 100

Random Posts
EXAMPLE:
Calculate the percent error from the previous examples.
Percent error = [( 36.08 – 36.10 )/ 36.08] x 100 = 0.06%
EXPERIMENTAL PROCEDURE FOR PERCENT WATER IN A HYDRATE
1. Accurately weigh a clean, dry evaporating dish. Record this mass.
2. Transfer approximately 3 grams of barium chloride dihydrate, BaCl2 ·2 H2O, into the weighed evaporating dish and weigh the dish and its contents. Record this mass.
3. Place the dish on a ring stand and heat gently for 10 minutes. Then heat the sample more strongly for 10 more minutes by bringing the flame of the bunsen burner directly under the dish. The residue should be almost pure white. Allow the dish to cool, then weigh it.
4. Heat the dish for another 5 minutes, cool, then weigh. If all the water has been driven off, the two masses should agree.
5. Dispose of the barium chloride in the container provided.
DATA SHEET FOR PERCENT WATER IN A HYDRATE
Name _________ Hood No. ______ Date _______
1. Mass of empty evaporating dish ____grams
2. Mass of dish & BaCl2 ·2 H2O ____grams
3. Mass of BaCl2 ·2 H2O ____grams (#2 – #1)
4. Mass of dish & BaCl2 after first heating ____grams
5. Mass of BaCl2 after first heating ____grams (#4 – #1)
6. Mass of dish & BaCl2 after second heating ____grams
7. Mass of BaCl2 after second heating ____grams (#6 – #1)
8. Mass of water lost ____grams
9. Percent hydration % ____%
CALCULATION OF PERCENT HYDRATION FROM THE CHEMICAL FORMULA
Calculate the percent hydration for barium chloride dihydrate, BaCl2 ·2 H2O(s).
Atomic masses:
H = 1.01; O = 16.00; Cl = 35.45; Ba = 137.33
Theoretical value _______%
PERCENT ERROR
Determine the percent error between the experimental value and the calculated value.
Percent error _______%
REMINDERS:
1. Remember that barium is toxic. The used barium chloride should be put in the waste container provided.
2. In determining the mass of water lost and the moles of anhydrous sample, the lower mass of the two should be used, this will usually be after the second heating, but if the mass after the first heating is lower, have them use that figure.
3. It is very important that the evaporating dish cools to room temperature before weighing. If it is not cool, convection currents will be set up that will lower the mass.
water
Water is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth’s hydrosphere and the fluids of all known living organisms (in which it acts as a solvent[1]). It is vital for all known forms of life, even though it provides no calories or organic nutrients. Its chemical formula is H2O, meaning that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. Two hydrogen atoms are attached to one oxygen atom at an angle of 104.45°.[2]


Hydrate
Water is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth’s hydrosphere and the fluids of all known living organisms (in which it acts as a solvent[1]). Its chemical formula is H2O, meaning that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. Two hydrogen atoms are attached to one oxygen atom at an angle of 104.45°.[2]

read more:




