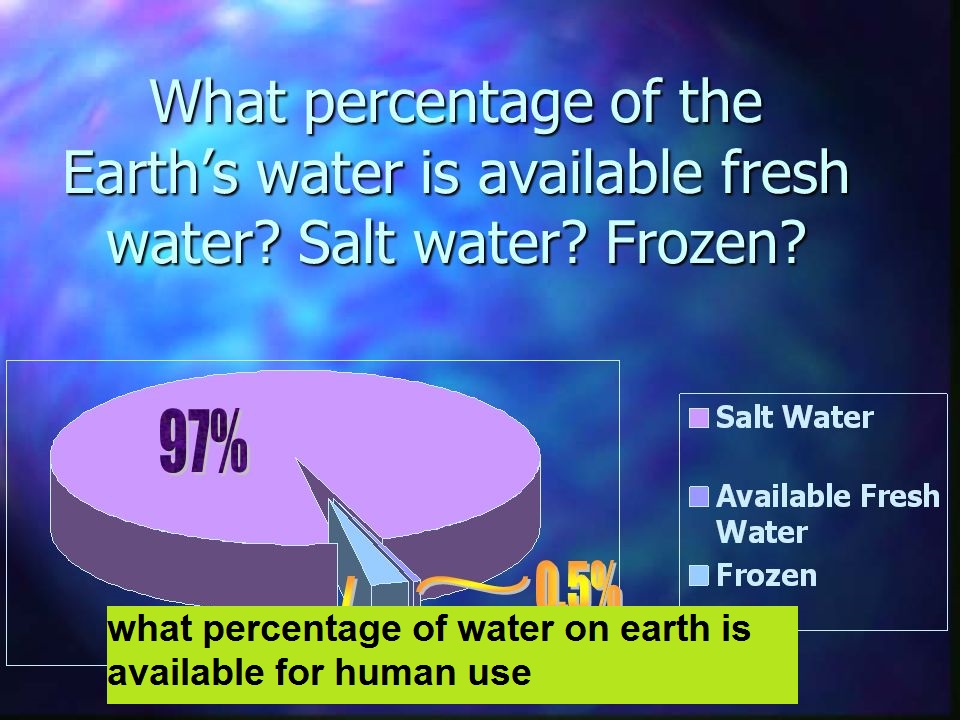
how to calculate percentage of water in hydrated salt
Hello dear friends, thank you for choosing us. In this post on the solsarin site, we will talk about “ how to calculate percentage of water in hydrated salt “.
Stay with us.
Thank you for your choice.

How can I calculate the percent composition of water in a hydrate?
This is a classic chem lab! It involves massing a compound, then heating it to remove water and then measuring its mass a second time.
Explanation:
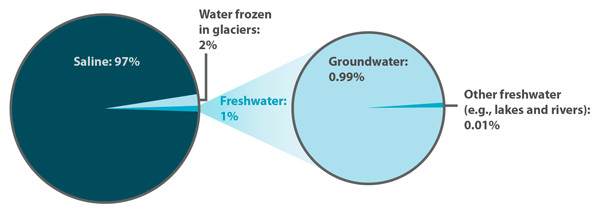

This can be approached in two different ways, calculating the percent of water in a hydrate from experimental data or analysis of a chemical formula.
Let’s discuss experimental analysis first. Let’s say that you are given a hydrated salt we’ll call Y. Its general formula would be…
Y•X(H2O).
calculate the percent of water
If you heat the sample you will be able to dry it and remove all of the water leaving just Y. To calculate the percent of water you will divide the change in mass of your sample (mass of water removed) by the mass of the hydrated salt (original mass).
If you are given the formula you would do analysis of the information provided…
CuSO4 = 159.62g
Mass of water = 18.02 x 5 = 90.1g
CuSO4•5(H2O) = 249.72g
% water = (90.1 / 249.72) x 100 = 36.08%
Presentation on theme: “Determining the Percent of Water in a Hydrated Salt Questions and Calculations Help.”— Presentation transcript:
Determining the Percent of Water in a Hydrated Salt Questions and Calculations Help
2 Calculation
#1a Use the % composition equation from your reference tables to solve this problem. Use the % composition equation from your reference tables to solve this problem. Mass of water = part Mass of water = part Mass of hydrated salt = whole Mass of hydrated salt = whole
3 Calculation
#1b Use the % composition equation from your reference tables to solve this problem. Use the % composition equation from your reference tables to solve this problem. Mass of anhydrous salt = part Mass of anhydrous salt = part Mass of
hydrated salt = whole Mass of hydrated salt = whole


4 Calculation
#2 a&b This question references the first question. If you had 100 grams of sample, use your percentages from #1 to determine grams of water and anhydrous salt would be present. See next slide… This question references the first question. If you had 100 grams of sample, use your percentages from #1 to determine grams of water and anhydrous salt would be present. See next slide…
5 Calculation
#2 a&b For example: For example: If you calculated 25% water and 75% anhydrous, then 25 grams would be water and 75 grams would be anhydrous salt. If you calculated 25% water and 75% anhydrous, then 25 grams would be water and 75 grams would be anhydrous salt.
6 Calculation
#3 Change grams of water (driven off) to moles of water. Change grams of water (driven off) to moles of water. Grams water moles water Grams water moles water Use “Mole Triangle” if necessary Use “Mole Triangle” if necessary
7 Calculation
4a Do the following calculation for your unknown only!!!! Do the following calculation for your unknown only!!!! Use the masses from the Periodic Table to determine the total mass for your unknown. (See #35 in your Unit VI note packet for additional help) Use the masses from the Periodic Table to determine the total mass for your unknown. (See #35 in your Unit VI note packet for additional help).
Calculation 4b Use the formula mass you just calculated to convert the mass of your anhydrous salt (from your data table) to moles. Use the formula mass you just calculated to convert the mass of your anhydrous salt (from your data table) to moles. Grams anhydrousmoles Grams anhydrousmoles Use “Mole Triangle” if needed. Use “Mole Triangle” if needed.
9 Calculation
5 Use the moles you calculated in #3 and #4b to determine the smallest whole number ratio. Use the moles you calculated in #3 and #4b to determine the smallest whole number ratio. Divide the larger # by the smaller #. Round the answer to a whole number. Divide the larger # by the smaller #. Round the answer to a whole number. This will give you “X” in the formula. Please re-write the formula and replace “X” with the # you just calculated. This will give you “X” in the formula. Please re-write the formula and replace “X” with the # you just calculated
What if there was water in the crucible when you started? Would this make you percent of water change?
(Increase, Decrease, or remain the same?) What if there was water in the crucible when you started? Would this make you percent of water change? (Increase, Decrease, or remain the same?)
11 Question 7
In this lab we re-heated the crucible & cover a second time to make sure the mass did not change. In this lab we re-heated the crucible & cover a second time to make sure the mass did not change. How might your percentage be affected if we had just heated once and assumed we removed all the water? How might your percentage be affected if we had just heated once and assumed we removed all the water?
12 Question 8
Inert material = material that does not react. Inert material = material that does not react. How would your % of water be affected if material was in the crucible when you began and remained there for the entire lab? How would your % of water be affected if material was in the crucible when you began and remained there for the entire lab?
13 Question 9 & 10
See your Unit VI notes (Pages 21-24) for help with these two questions. See your Unit VI notes (Pages 21-24) for help with these two questions. #9 – You are calculating an empirical formula. #9 – You are calculating an empirical formula. #10 – You are calculating a molecular formula. #10 – You are calculating a molecular formula.