periodic table number meanings
Hello dear friends, thank you for choosing us. In this post on the solsarin site, we will talk about “periodic table number meanings”.
Stay with us.
Thank you for your choice.
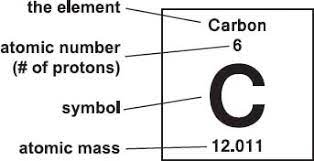
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.

What is the periodic table?
The periodic table is a classification system for the elements. Each element has unique properties and you can predict an element’s physical and chemical properties based on its position in the periodic table.
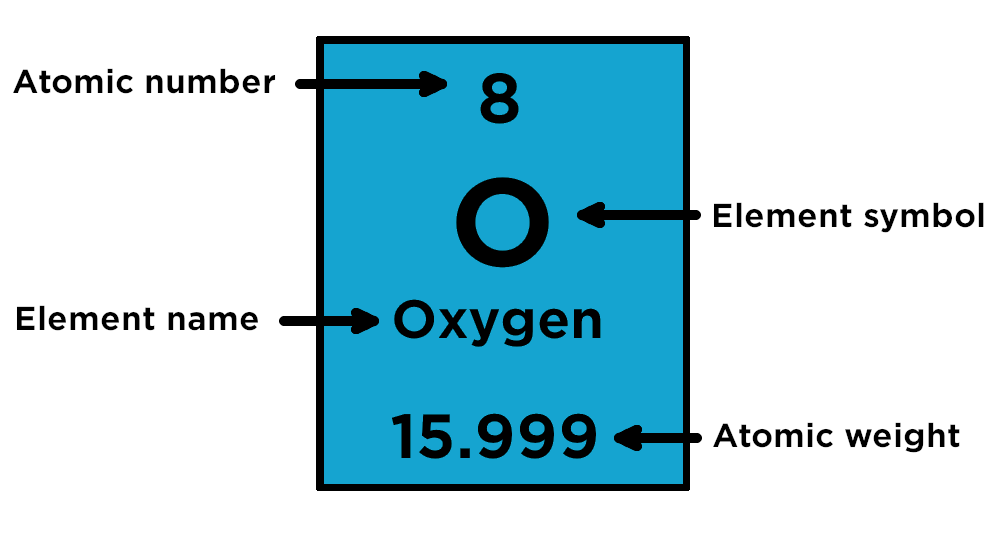
What do the symbols and numbers mean?
When you look at the periodic table, the symbols and numbers look like the image on the left. The symbols are abbreviations of the origins of the word for each element. For example, the origin of lead’s symbol, Pb, is the Latin word ‘plumbum’ meaning ‘liquid silver’‘.
The number above the symbol is the atomic mass (or atomic weight). This is the total number of protons and neutrons in an atom.
The number below the symbol is the atomic number and this reflects the number of protons in the nucleus of each element’s atom. Every element has a unique atomic number. Lead has 82 protons therefore its atomic number is 82. The elements are then arranged in order of increasing atomic numbers – so hydrogen (H), which only has one proton, is located first in the table, while sodium (Na), which has 11 protons, and is found further down the table.
We can use atomic number and atomic mass to find out the number of protons, neutrons and electrons there are in an atom. By subtracting the atomic number from the atomic mass we can discover the number of neutrons there are.
MORE POSTS:
- who did lee krasner marry?
- how much alcohol is in smirnoff watermelon vodka
- what event symbolized the end of communism?
- primary succession in a sentence
- what year was the sandlot released?
How does it work?
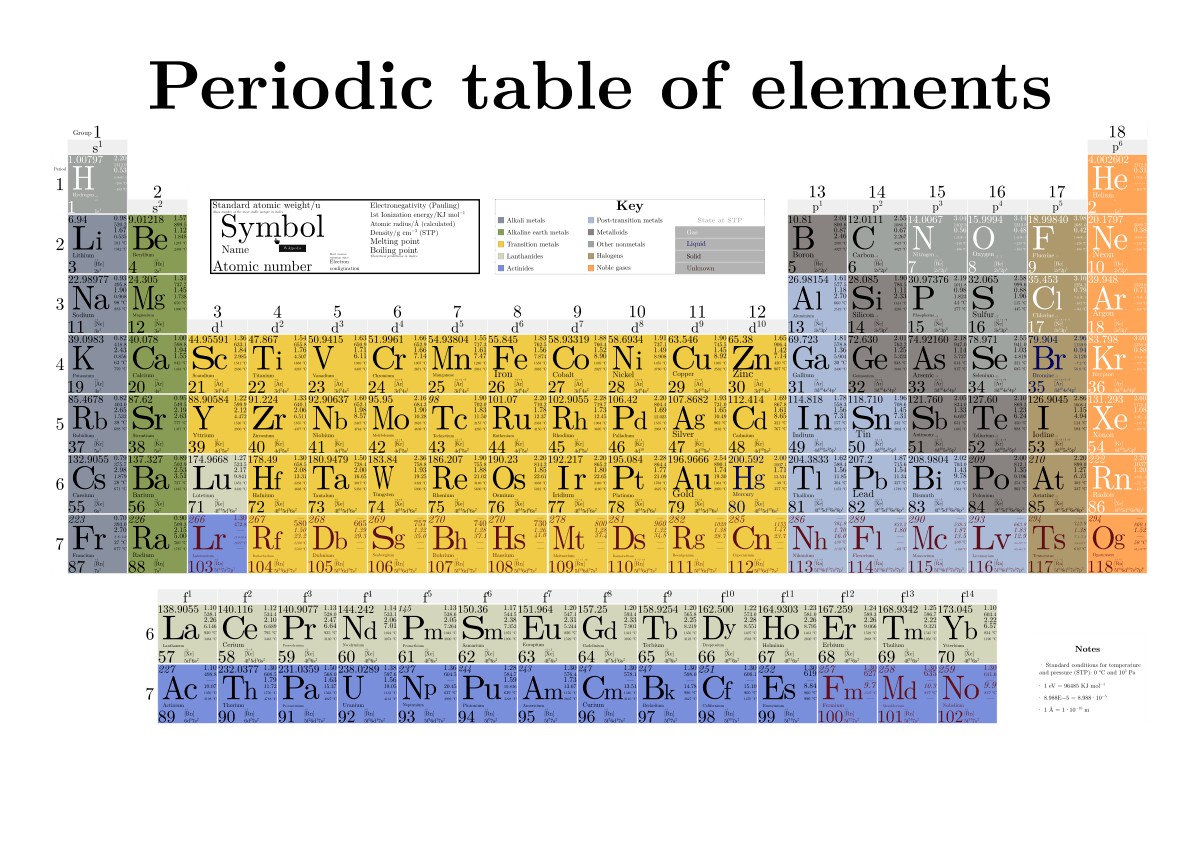
There are 18 main columns of elements in the periodic table. These are called groups or families. Elements within the same group or family have similar – but not identical – properties. For example lithium, sodium and potassium are members of family 1. They are all highly reactive, and thus can easily combine with other elements to form compounds. Each horizontal row is called a period. Unlike elements in a family, elements in a period are not alike in properties. There are 7 periods of elements. The 2 rows that have been separated out of the main table are rare-earth elements, or lanthanons (lanthanides) in the first period and actinons (actinides) in the next period.
Who invented it?
The journey towards the creation of the periodic table can be traced as far back as the Ancient Greeks of 400BC who used the words “element” and “atom” to describe the differences between things.
The periodic table we know today was created by 4 separate European scientists during the 1860s. In 1863, 44 year-old French geologist A. E. Béguyer de Chancourtois created a list of elements measured by their atomic weight. Meanwhile, in England, chemist John A. R. Newlands noticed that chemical groups repeated every 8 elements. He named this the octave rule, comparing it to musical notes.
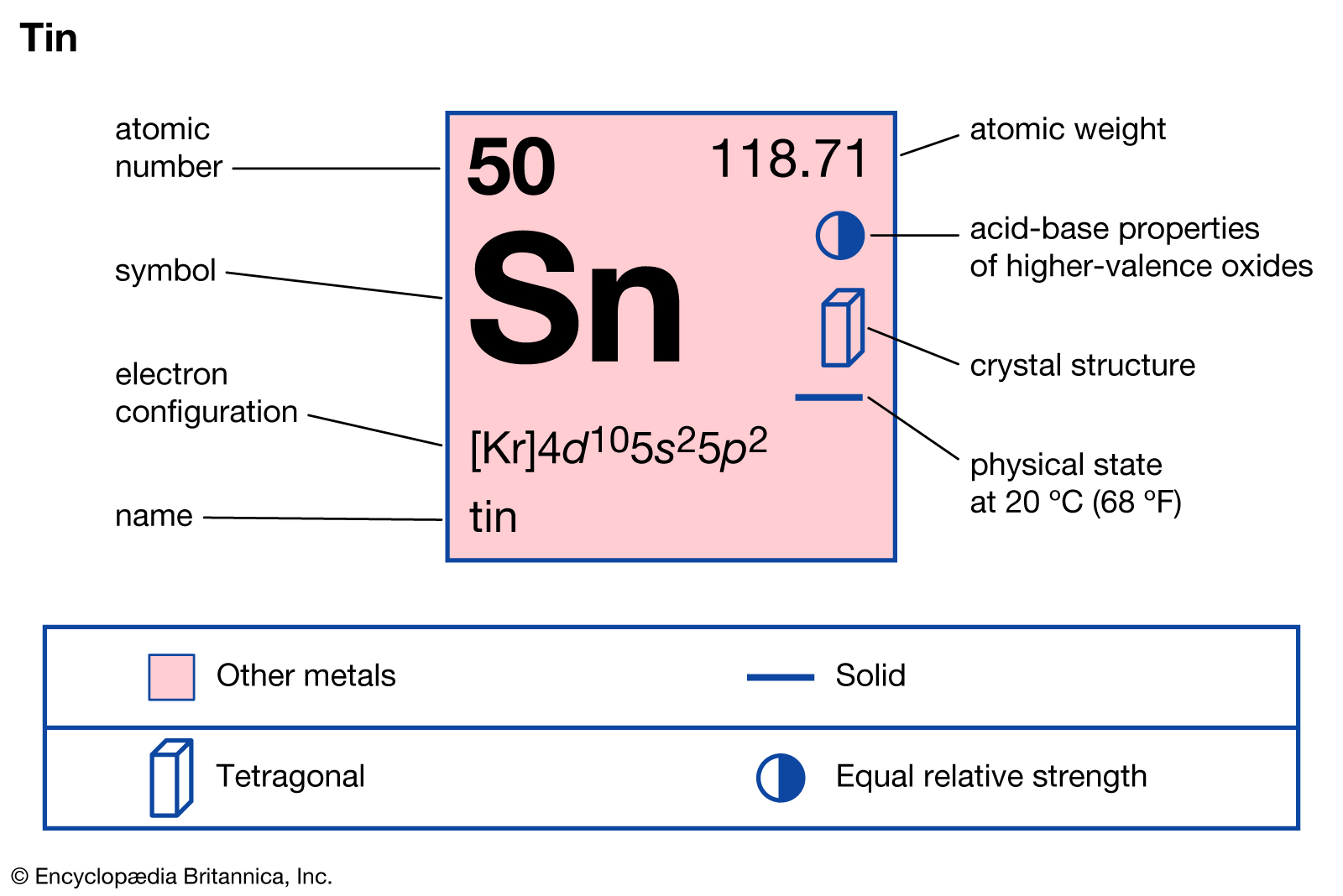

Between 1868 and 1869, working independently, Russian chemist Dmitri I. Mendeleev and German chemist Lothar Meyer arranged the elements into 7 columns relating to their chemical properties. Their tables were similar, but it was Mendeleev’s table that was adopted. It assembled observations and discoveries of earlier chemists, showing the symbols of the chemical elements in the order of their atomic masses, but his table was a huge scientific breakthrough because he left gaps and accurately predicted undiscovered elements.
This greatly accelerated the discovery of new elements and our understanding of their properties, and it enabled scientists to accurately predict the elements – many elements have been discovered or artificially made which have fitted into the gaps in the table. Mendeleev predicted the properties of scandium, gallium and germanium: within 20 years scientists had discovered the existence of these elements and his predictions were found to be accurate. Modern versions of Mendeleev’s table have 109 elements, but new elements are being discovered all of the time.
How to Read the Periodic Table
Each row on the table is called a period. All the elements in a period have the same number of orbitals. This starts from one orbital at the top row, to seven orbitals at the bottom row. Each row increases by one orbital. Elements in the same period have similar physical properties.
Each column on the table is called a group. All the elements in each group have the same number of electrons in their outer shell, also called valence electrons. Elements in the same group react with other elements in similar ways.
The atomic number, the number at the top of each element box, is the number of protons in the nucleus and the number of electrons in the atom.
The elemental symbol is an abbreviation of the element name. Some periodic tables do not include the element names, only the symbols.
The atomic weight is the average mass of all the element’s isotopes. It is the average number of all the protons and neutrons in the nucleus. Generally, the atomic mass increases as you move down or across each group or period. Of course, there are exceptions to this rule.
There are primarily three ways that people navigate the periodic table: group, period, and atomic number.

Groups
The columns of the periodic table are called groups. It may seem strange to call columns groups, but this is done because elements of the same group have similar properties. In fact, the periodic table organizes elements into groups based on their properties. This is one reason for the strange shape of the table. The group number is listed on top of each column. The table shown above shows 18 groups. Some tables only show eight groups, while others show up to 32 groups. 18 groups is the most common, but the number of groups does not have a significant impact on the information the table provides.
Periods
The rows of the periodic table are called periods. This may seem silly, but let’s think about why they are named periods for a bit. When we think about waves, orbits, circular motion, or really anything that repeats, the time that it takes to do one cycle is called a period. Examine the sine wave depicted below.
At every interval of 1 (0−1, 1−2, 2−3, etc), the graph of the sine wave exactly repeats itself. The sine wave is said to be periodic with an interval of 1. Well, the periodic table is periodic through the properties of the elements. If we go across a period of the table from the left to the right, the properties of the elements change drastically until we get to the end of the row. The next element is directly below the element we started at. This means they are in the same group. Think back to what we learned about groups: elements in the same group have similar properties. This means these two elements have similar properties. As we can see, the cycle of properties repeats itself with every period.
The periods are numbered on the left side of each row. There are seven periods overall. The two rows on the bottom actually belong in periods 6 and 7. They are typically taken out like this in order to improve the readability of the table.
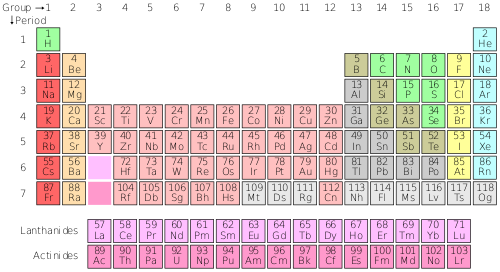

Atomic number
The atomic number is the number of protons an element has. The atomic number increases by one as we move one element to the right. That is to say, if an element has an atomic number of 25, the element directly on its right will have the atomic number 26. When we reach the end of a period, the next element is the leftmost element of the next period.