law of conservation of matter
Hi, welcome to solsarin site, in this post we want to talk about“law of conservation of matter”,
stay with us.
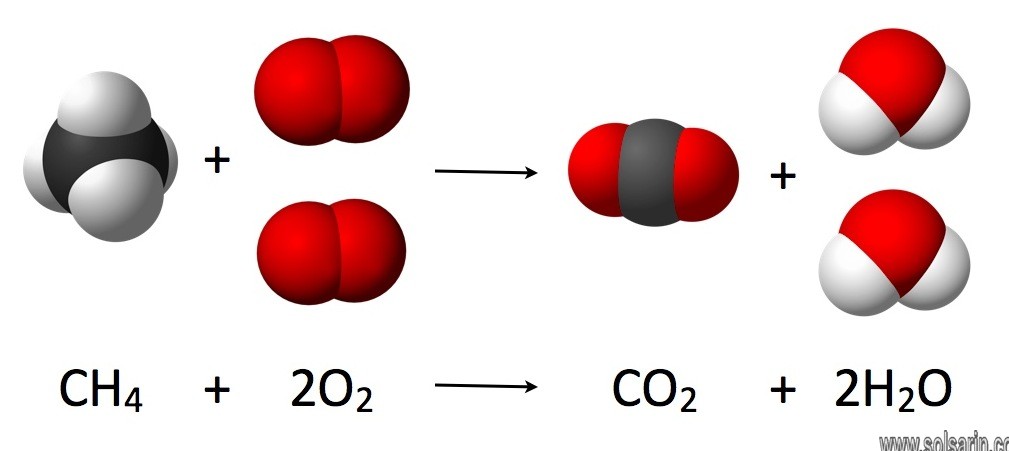

In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system’s mass cannot change, so quantity can neither be added nor be removed.
What does the law of conservation of matter?
The Law of Conservation of Matter states that matter cannot be created or destroyed. In a physical change, substances can change form, but the total mass remains the same. In a chemical change, the total mass of the reactants always equals the total mass of the products.
What is the law of conservation of mass of matter?
The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. For example, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change.
Which statement best describes the law of conservation of matter?
According to the law of conservation of matter, matter is neither created nor destroyed, so we must have the same number and type of atoms after the chemical change as were present before the chemical change.
What does the law of conservation of matter state ?
Law of Conservation of Matter. States that, during a chemical reaction, matter cannot be created or destroyed. Even though the matter may change from one form to another, the same number of atoms exists before and after the changes take place. reactant.
What is the law of conservation of matter kids?
The Law of Conservation of Matter says that the amount of matter stays the same, even when matter changes form. Sometimes it may seem that matter disappears during a science experiment, but this law tells us that matter cannot magically appear or disappear, it simply changes from one form to another.
Why is the law of conservation of matter important?
According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants. The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses, such the amount of gas consumed or produced during a reaction.
What does the law of conservation of matter state Brainly?
Answer: The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system’s mass cannot change, so quantity can neither be added nor be removed.
Do you want to know about “an empty-kcalorie food is one that contains“? Click on it.
What is the law of conservation of mass give an example?
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.
What is the law of conservation of mass ?
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants. Reactants.
How is the law of conservation of matter related to balancing equations explain?
The law of conservation of matter states that no atoms are created or destroyed in a chemical reaction. Therefore, a balanced chemical equation will show the same number of each type of atom on each side of the equation.


What is the law of conservation of mass 5th grade?
5 Experiments of Physical and Chemical Changes. The Law of Conservation of Mass states that mass is conserved in physical and chemical changes. Students explore this concept by taking initial masses, making predictions, and finding final masses of physical and chemical changes.
How do you prove the law of conservation of matter?
The amount of matter is conserved when a substance changes form. When matter changes drastically it is not actually destroyed. This can be tested by weighing all the materials involved in an experiment before starting it, and again after the experiment.
What does the law of conservation of matter show apex?
What does the law of conservation of matter show apex? States that, during a chemical reaction, matter cannot be created or destroyed. Even though the matter may change from one form to another, the same number of atoms exists before and after the changes take place.
What is law of conservation of matter Class 9?
Class 9 Chemistry Atoms and Molecules. Laws of conservation of mass. Laws of conservation of mass. The law states that mass can neither be created nor destroyed in a chemical reaction i.e. Total masses of reactants is equal to the sum of masses of products and the masses of unreacted reactants.
What is the law of conservation of mass give an example class 9?
So, in a chemical reaction, the total mass of reactants must be equal to the total mass of products. Example: When calcium carbonate is heated, a chemical reaction takes place to form calcium oxide and carbon dioxide.
What scientific principle state of matter is neither created nor destroyed is chemical process?
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
What has chemistry ever done for you?
The industrial applications of chemistry directly affect our daily lives—what we eat, what we wear, our transport, the technology we use, how we treat illnesses and how we get electricity—to name just a few. Research is constantly deepening our understanding of chemistry, and leading to new discoveries.
How is matter described?
Matter is anything that has mass and takes up space. Matter can be described in terms of physical properties and chemical properties. Physical properties and chemical properties of matter can change. Matter is composed of elements and compounds. Combinations of different substances are called mixtures.
How does the law of conservation of matter relate to life science?
The law of conservation of matter and energy states that matter is neither created nor destroyed but conserved. Humans do not have the ability to create or destroy matter (atoms) or energy. They can only rearrange the matter and energy. For example, an oxygen atom will cycle through a living system.
What happens when a liquid becomes a gas apex?
Answer: When a liquid changes to gas, the particles absorb heat energy and they start vibrating faster and distance between them increases. On constant vibration, intermolecular forces decrease and particles start moving away from each other and change to gas.
Can we create matter?
In the standard model, it is not possible to create a net amount of matter particles—or more precisely, it is not possible to change the net number of leptons or of quarks in any perturbative reaction among particles.
Can matter be made from nothing?
Under just the right conditions — which involve an ultra-high-intensity laser beam and a two-mile-long particle accelerator — it could be possible to create something out of nothing, according to University of Michigan researchers.
How does this equation represent the law of conservation of matter?
Matter cannot be created or destroyed in chemical reactions. This is the law of conservation of mass. In every chemical reaction, the same mass of matter must end up in the products as started in the reactants.

Have you heard anything about “how much is the coca-cola brand worth?“? Click on it.
Is water a matter?
States of Matter
Not only is water the most common substance on earth, but it is also the only substance that commonly appears as a solid, a liquid, and a gas within the normal range of earth’s temperatures. This makes water a good model for discussing the solid, liquid, and gas states of matter.
Is light a matter?
Light is a form of energy, not matter. Matter is made up of atoms. Light is actually electromagnetic radiation. Moving electric charge or moving electrons (electric current) cause a magnetic field, and a changing magnetic field creates an electric current or electric field.

Is matter just energy?
So energy and matter are really the same thing. Completely interchangeable. And finally, Although energy and mass are related through special relativity, mass and space are related through general relativity.
random posts:



