what causes a water molecule to be polar?
Hello friends! Do you know about “what causes a water molecule to be polar“? So stay tuned to solsarin.
Water (H2O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the “universal solvent” and the “solvent of life.”
It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth’s surface. It is also the third most abundant molecule in the universe (behind molecular hydrogen and carbon monoxide).
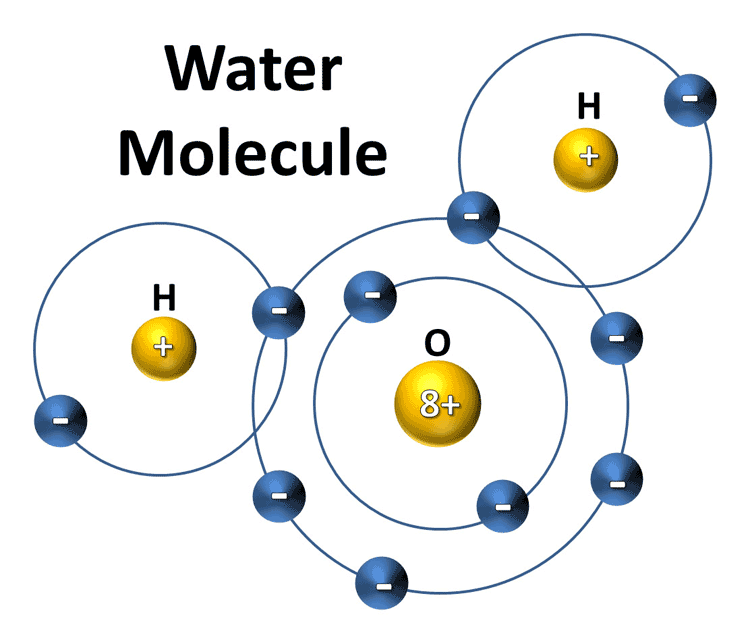

Water Molecule
Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form, a relatively high boiling point of 100 °C for its molar mass, and a high heat capacity.
Water is amphoteric, meaning that it can exhibit properties of an acid or a base, depending on the pH of the solution that it is in; it readily produces both H +and OH−ions.
Related to its amphoteric character, it undergoes self-ionization. The product of the activities, or approximately, the concentrations of H +and OH− is a constant, so their respective concentrations are inversely proportional to each other.
Physical properties
Water is the chemical substance with chemical formula H
2O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Water is a tasteless, odorless liquid at ambient temperature and pressure.
Liquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. This can easily be observed in a water-filled bath or wash-basin whose lining is white. Large ice crystals, as in glaciers, also appear blue.
Under standard conditions, water is primarily a liquid, unlike other analogous hydrides of the oxygen family, which are generally gaseous. This unique property of water is due to hydrogen bonding. The molecules of water are constantly moving concerning each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (2 × 10−13 seconds).
However, these bonds are strong enough to create many of the peculiar properties of water, some of which make it integral to life.
Water is a polar molecule and also acts as a polar solvent.The positive charge comes from the atomic nucleus, while the electrons supply the negative charge. It’s the movement of electrons that determines polarity. Here’s how it works for water.
Polarity and hydrogen bonding
An important feature of water is its polar nature. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The oxygen atom also has two lone pairs of electrons. One effect usually ascribed to the lone pairs is that the H–O–H gas-phase bend angle is 104.48°, which is smaller than the typical tetrahedral angle of 109.47°.
The lone pairs are closer to the oxygen atom than the electrons sigma bonded to the hydrogens, so they require more space. The increased repulsion of the lone pairs forces the O–H bonds closer to each other.
Another consequence of its structure is that water is a polar molecule. Due to the difference in electronegativity, a bond dipole moment points from each H to the O, making the oxygen partially negative and each hydrogen partially positive.
hydrogen bonding
A large molecular dipole, points from a region between the two hydrogen atoms to the oxygen atom. The charge differences cause water molecules to aggregate (the relatively positive areas being attracted to the relatively negative areas). This attraction, hydrogen bonding, explains many of the properties of water, such as its solvent properties.
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for several of the water’s physical properties.
These properties include its relatively high melting and boiling point temperatures: more energy is required to break the hydrogen bonds between water molecules. In contrast, hydrogen sulfide (H2S), has much weaker hydrogen bonding due to sulfur’s lower electronegativity. H2S is a gas at room temperature, despite hydrogen sulfide having nearly twice the molar mass of water.
The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.

Cohesion and adhesion
Water molecules stay close to each other (cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high adhesion properties because of its polar nature. On clean, smooth glass the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces. In biological cells and organelles, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a strong attraction to water.
Irving Langmuir observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less. They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.
Surface tension
Water has an unusually high surface tension of 71.99 mN/m at 25 °C which is caused by the strength of the hydrogen bonding between water molecules. This allows insects to walk on water.
Capillary action
Because water has strong cohesive and adhesive forces, it exhibits capillary action. Strong cohesion from hydrogen bonding and adhesion allows trees to transport water more than 100 m upward.


The Polarity of Water
The polar nature of water is a particularly important feature that contributes to the uniqueness of this substance. The water molecule forms an angle with an oxygen atom at the vertex and hydrogen atoms at the tips. Because oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge.
An object with such a charge difference is called a dipole (meaning “two poles”). The oxygen end is partially negative, and the hydrogen end is partially positive; because of this, the direction of the dipole moment points from the oxygen toward the center position between the two hydrogens. This charge difference causes water molecules to be attracted to each other (the relatively positive areas are attracted to the relatively negative areas), as well as to other polar molecules.
This attraction contributes to hydrogen bonding and explains many of water’s properties (including its ability to act as a solvent to many substances).
The Polarity
Polarity of the water moleculeOwing to the electronegativity difference between hydrogen (H) and oxygen (O) atoms, and the bent shape of the H2O molecule, a net dipole moment exists. The figure indicates the partial charges that the atoms possess.
A water molecule can form a maximum of four hydrogen bonds. It can form by accepting two hydrogen atoms and donating two hydrogen atoms.
One such property is its relatively high melting and boiling points; more energy is required to break the hydrogen bonds between molecules in order to change to a higher energy phase.
Polarity of a Water Molecule
Water (H2O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen is on side of the molecule. And the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding. When solutes are added to water, they may be affected by the charge distribution.
The reason the shape of the molecule isn’t linear and nonpolar (e.g., like CO2) is because of the difference in electronegativity between hydrogen and oxygen. The electronegativity value of hydrogen is 2.1, while the electronegativity of oxygen is 3.5.
The water Molecule
The smaller the difference between electronegativity values, the more likely atoms will form a covalent bond. Hydrogen and oxygen are both acting as nonmetals under ordinary conditions. But oxygen is quite a bit more electronegative than hydrogen. So the two atoms form a covalent chemical bond, but it’s polar.
Remember that even though the covalent bond between each hydrogen. And oxygen in water is polar, a water molecule is an electrically neutral molecule overall. Each water molecule has 10 protons and 10 electrons, for a net charge of 0.
Why Water Is a Polar Solvent
The shape of each water molecule influences the way it interacts with other water molecules and with other substances. Water acts as a polar solvent.
The slight negative charge near the oxygen atom attracts nearby hydrogen atoms from water or positive-charged regions of other molecules. The slightly positive hydrogen side of each water molecule attracts other oxygen atoms and negatively-charged regions of other molecules. The hydrogen bond between the hydrogen of one water molecule and oxygen of another holds water together. And it gives it interesting properties, yet hydrogen bonds are not as strong as covalent bonds.


The polarity of water
Many other unique properties of water are due to the hydrogen bonds. For example, ice floats because hydrogen bonds hold water molecules further apart in a solid than in a liquid, where there is one less hydrogen bond per molecule. The unique physical properties, including a high heat of vaporization, strong surface tension, high specific heat.
And nearly universal solvent properties of water are also due to hydrogen bonding. The hydrophobic effect is particularly important in the formation of cell membranes. The best description is to say that water “squeezes” nonpolar molecules together.
Water’s Polarity
The two hydrogen atoms and one oxygen atom within water molecules (H2O) form polar covalent bonds. While there is no net charge to a water molecule, the polarity of water creates a slightly positive charge on hydrogen.
And a slightly negative charge on oxygen, contributing to water’s properties of attraction. Since water is a nonlinear, or bent, molecule, the difference in electronegativities between the oxygen. And hydrogen atoms generates the partial negative charge near the oxygen and partial positive charges near both hydrogens.
As a result of water’s polarity, each water molecule attracts other water molecules. It is because of the opposite charges between them, forming hydrogen bonds. Water also attracts, other polar molecules and ions, including many biomolecules. For example sugars, nucleic acids, and some amino acids.
In contrast, nonpolar molecules, such as oils and fats, do not interact well with water, as shown in. These molecules separate from it rather than dissolve in it, as we see in salad dressings containing oil and vinegar.
Thanks for taking the time to this post “what causes a water molecule to be polar?”.




