NH4 hydrogen bonding
Hello guys! We return with an amazing topic. It is about “NH4 hydrogen bonding”. This topic will be placed in the Scientific category on solsarin‘s site. If you are interested in such topics follow us.
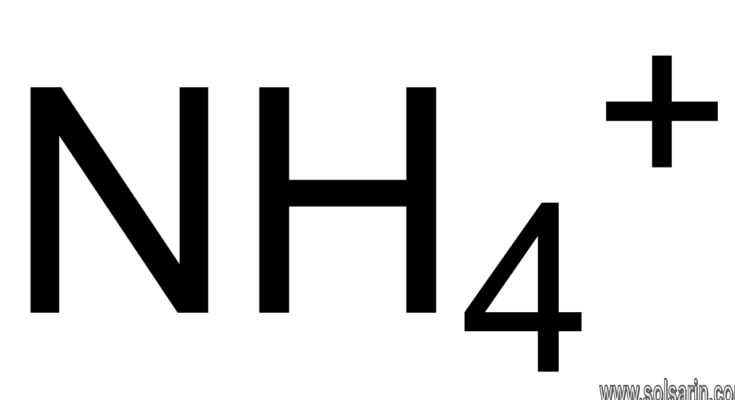

Hydrogen bond
A hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group, and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn–H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F).
Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms, and can vary between 1 and 40 kcal/mol.
This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins. Hydrogen bonds are responsible for holding such materials as paper and felted wool together, and for causing separate sheets of paper to stick together after becoming wet and subsequently drying.
The hydrogen bond is responsible for many of the anomalous physical and chemical properties of compounds of N, O, and F. In particular, intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group-16 hydrides that have much weaker hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.
Why isn’t the ion NH4+ capable of hydrogen bonding?
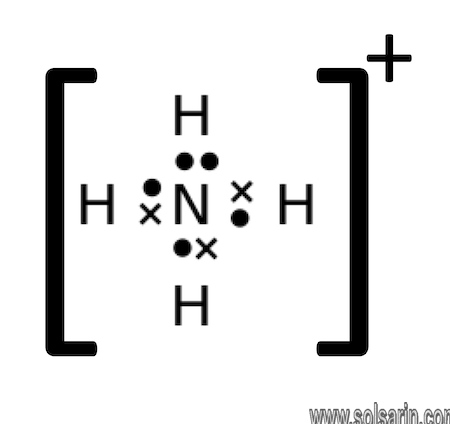
If you draw Lewis structure, central atom (N) will have 1 lone pair and 4 single bonds with Hydrogen. Why can’t hydrogen in this ion molecule be able to form hydrogen bonding with other molecules alike? Is it because there’s only 1 electron on the central atom? See for yourself: 5N + 4H -1 = 9 electrons to go around. 4 bonds = 8 electrons 9 – 8 electrons = 1 electron left. So this 1 electron is placed on the central atom.

Why can’t NH4+ form hydrogen bonds?
NH+4NH4+ can form hydrogen bonds — just not to itself, because it can only donate hydrogen bonds, not accept them.
Note: a hydrogen bond donor is one that has hydrogen atoms that are Lewis acids; a hydrogen bond acceptor is a Lewis base. Ammonium ion is not a Lewis base; it has no available electron pairs to function that way.
Hydrogen bonding of ammonium ion and ???
Our study case is ammonium in water, NH4^+ (aq), which forms stronger hydrogen bonds with water than does neutral NH3.” The authors go on to say, “The NH+4⋯H2O system is stabilized dominantly by a strong ion – dipole interaction.
Therefore, it would appear that ammonium does form hydrogen bonds with water. But you may be referring to NH4^+⋯NH4^+ hydrogen bonding, probably in comparison to hydrogen bonds between ammonia molecules, NH3⋯NH3.
NH4+ Lewis Structure and Hybridization
NH3 is the chemical formula of Ammonia. A positively charged polyatomic ion of Ammonium or NH4+ comes into existence when an Ammonia atom goes through the process of protonation, that is, it loses one of its electrons and becomes positively charged.
A protonated Ammonium ion or NH4+ is made up of Nitrogen and Hydrogen. The ion is the by-product of a chemical reaction between a proton donor and Ammonia, which is as follows:
NH3 + H+ ——> NH4+
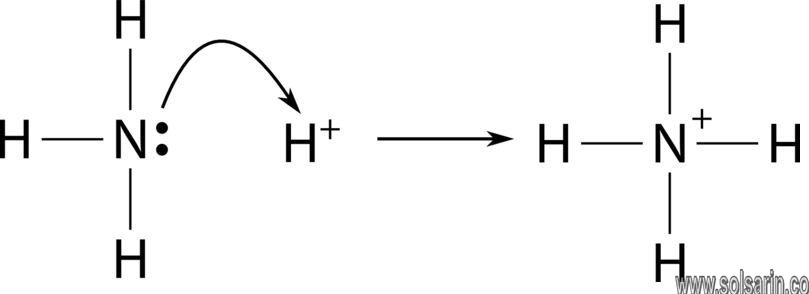

What is the Lewis Structure of NH4?
As mentioned earlier, NH4+ is made up of Nitrogen and Hydrogen. If we look towards the periodic table, we will find Hydrogen in group 1.
This means that Hydrogen has 1 electron. NH4+ has 4 hydrogen atoms, therefore, there are 4 hydrogen electrons.
Nitrogen’s valence electron count, however, is 5, owing to its position in the 5th group of the periodic table. The plus sign denotes the absence of 1 electron; therefore, it is minus one.
If we total out the number of electrons, it will be (1×4) + (5×1) – 1 = 4 + 5 – 1 = 8.
We thus have 8 valence electrons here.
Keeping Nitrogen in the center, and considering Hydrogen’s position on the outside, we can place the 4 hydrogen atoms surrounding the single nitrogen atom.
Next is putting the chemical bonds.
Since the NH4+ atom has 8 valence electrons, our arrangement will be according to 2,4,6, and 8.
Referring to the octet rule, hydrogen needs only 2 valence electrons, which it already has. Even Nitrogen, which needs 8 electrons in the valence shell has all 8 of them, thereby forming a full exterior shell.
The loss of an electron is depicted by putting a + sign enclosing the Lewis structure.
The Hybridization of NH4
The concept of Hybridization decrees that atomic orbits fuse with one another to form new degenerated hybrid orbitals, which influence bonding properties and molecular geometry of the atoms of an element.
It can be considered as an extension of the valence bond concept and lays its foundation on the molecular and quantum mechanics of an atom.
These new orbitals may have different shapes, energies, etc. when compared to the previous ones. Hybridization brings about changes in the orbital arrangement of an atom as well.
Such a structure arises from the need for a refined geometry of atoms necessary for electrons to pair up and thus, form different chemical bonds, as inducted by the valence bond theory.
These hybrid orbitals, formed by the hybridization of an atom, are helpful in the explanation and understanding of an atom’s molecular geometry, its atomic bond properties, and the position in the atomic space.
In most common scenarios, atomic orbitals with similar energy combine to form hybrid orbitals.
While the exchange between atomic orbits of different atoms leads to the creation of molecular orbits, hybridization of an atom is assumed to be a combination of different atomic orbits, overlaying one another in different fractions.
Atomic orbits of comparable levels of energy participate in forming hybrid orbitals. This process can also involve half-filled and fully filled orbitals as well, provided that the level of energy remains similar.
During hybridization, the orbitals having similar energy can mix. Most common types of hybridizations are sp, sp2, sp3, sp3d, sp3d2, sp3d3 etc.
Ammonium ion formed by the release of an electron has 8 total electrons in the valence shell.
In NH4+, nitrogen and the 4 hydrogen atoms make 4 sigma bonds, out of which 3 are covalent bonds and the fourth one is a dative bond. The NH4+ ion has no pi bonds.
As a result, all four electrons contained in the atomic orbitals in the outermost shell of the nitrogen atom can participate in hybridization, making it SP3.
Another way of identifying the hybridization of an atom is by the following formula:
Hybridization = Number of Ion Pairs + Number of Sigma Bonds.
Since Ammonium has 0 ion pairs and 4 sigma bonds, the hybridization value is 4. Therefore, the configuration of NH4+ is SP3.

Hydrogen Bond-Assisted Ultra-Stable and Fast Aqueous NH4+ Storage
Aqueous ammonium ion batteries are regarded as eco-friendly and sustainable energy storage systems. And applicable host for NH4+ in aqueous solution is always in the process of development. On the basis of density functional theory calculations, the excellent performance of NH4+ insertion in Prussian blue analogues (PBAs) is proposing, especially for copper hexacyanoferrate (CuHCF). In this work, we prove the outstanding cycling and rate performance of CuHCF via electrochemical analyses, delivering no capacity fading during ultra-long cycles of 3000 times and high capacity retention of 93.6% at 50 C.
One of main contributions to superior performance from highly reversible redox reaction and structural change is verified during the ammoniation /de- ammoniation progresses. More importantly, we propose the NH4+ diffusion mechanism in CuHCF based on continuous formation and fracture of hydrogen bonds from a joint theoretical and experimental study, which is another essential reason for rapid charge transfer and superior NH4+ storage. Lastly, a full cell by coupling CuHCF cathode and polyaniline anode is constructing to explore the practical application of CuHCF. In brief, the outstanding aqueous NH4+ storage in cubic PBAs creates a blueprint for fast and sustainable energy storage.
Nh4+ Bond Angle
Four electron pairs arrange themselves in space in what is calling a tetrahedral arrangement. Also what is the bond angle of ammonia.
Nh4+ bond angle. Since NH4 is a cation the bond angle between 2 respective hydrogen atoms is 1095 degrees instead of 90 degrees which is as far away from one another as possible. Sp3 and 1095 sp3 and 180 D. The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory VSEPR theory with an experimentally determined bond angle of 1067.
Favorite Answer NH4 is indeed tetrahedral but the angle between bonds isn’t 90 degrees. Why is the H-N-H angle in NH4 identical to the H-C-H bond angle in CH4. The electron geometry for the.
Although the bond angle should be 1095 degrees for trigonal pyramidal molecular geometry it decreases to 107 degrees due to the lone pair on the nitrogen atom. Computed by PubChem 21 PubChem release 20190618. All the bond angles are 1095.
This is the arrangement of atoms in any tetrahedral molecule where four of the same. NH4 Bond Angle The bond angle of ammonium ion NH4 is 1095 degrees The bond angle of NH4 is greater than NH3 because the arrangement of NH4 is tetrahedral and the NH3 arrangement is different because three hydrogens surround the nitrogen. NH2- NH3 and NH4 have H-N-H bond angles of 105 107 and 109.