is baking soda a compound?
Hi,welcome to solsarin site, today we want to talk about“is baking soda a compound”,
thank you for choosing us.
is baking soda a compound?
Baking soda is a compound because it consists of different kinds of atoms (Sodium, Hydrogen, Carbon, and Oxygen) that bond chemically.
In Chemistry, a compound is a matter that is made up of two or more kind of atoms which bond chemically.
Because the atoms bond chemically, it’s difficult or impossible to break them down using a physical process.
So, if you want to separate a compound, you need to conduct a chemical reaction.
Can you break down baking soda?
Yes, you can break down baking soda or Sodium Bicarbonate using a chemical process called THERMAL DECOMPOSITION.
In thermal decomposition, baking soda breaks down into Sodium Carbonate (Na2CO3) a.k.a. washing soda, gaseous water (H2O), and gaseous Carbon dioxide (CO2) are produced.
The easiest example of baking soda decomposition is when baking a cake batter that contains baking soda. The gaseous water and carbon dioxide (of the baking soda) will escape and leaving the solid sodium carbonate (washing soda).


What is Baking Soda?
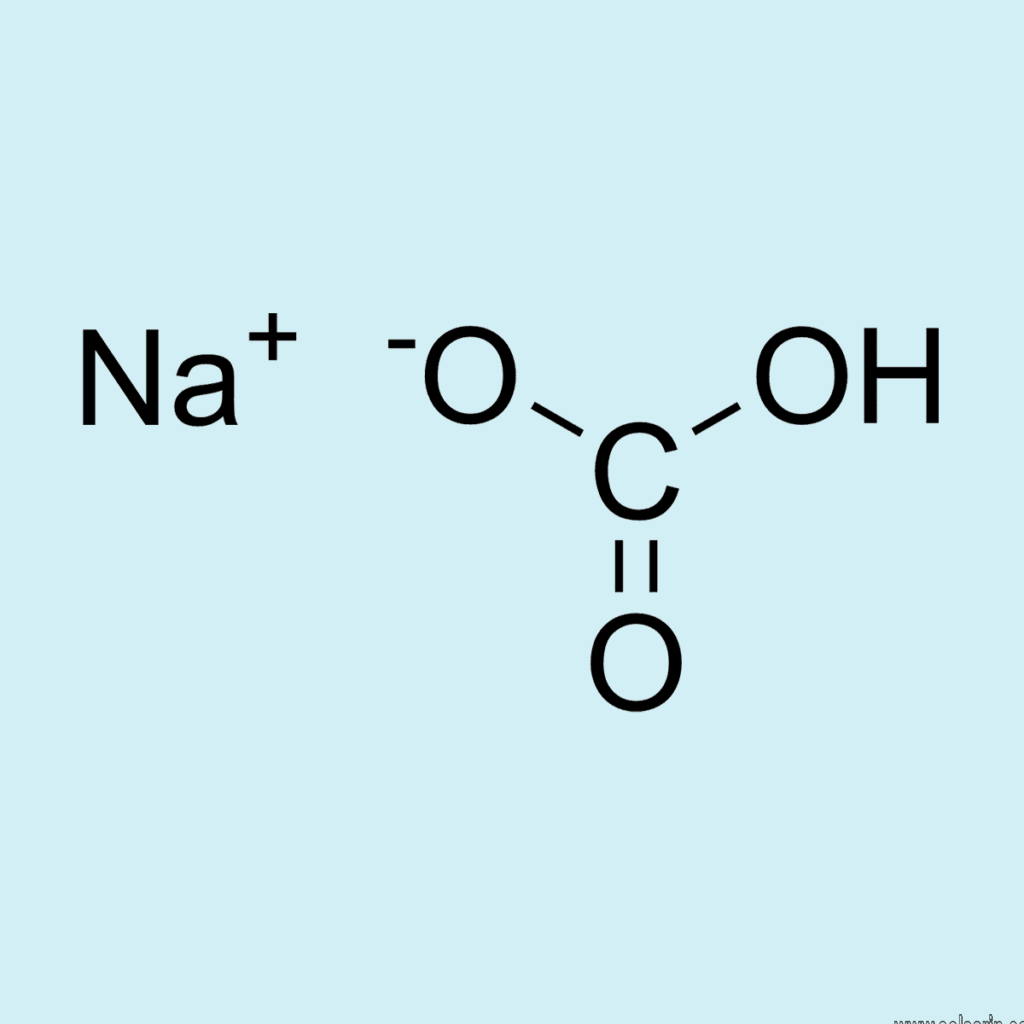
It is nothing but sodium bicarbonate, which can be denoted by the chemical formula NaHCO3. Baking soda is basically a salt that comprises a sodium cation which can be represented as Na+ and a bicarbonate anion which can be represented as HCO3−.
Baking soda is a white, crystalline solid that has a salty taste. moreover, It is quite useful in baking as a leavening agent – a substance that causes foaming, leading to the softening of the mixture.
Baking soda is a leavening ingredient that is commonly used in baked products such as cakes, muffins, and cookies.moreover, It’s a white crystalline powder that’s inherently alkaline, or basic. It’s also known as sodium bicarbonate.
How is Baking Soda a Compound?
We have already discussed that baking soda is a compound but, what are the characteristics that actually make it a compound.
The atoms of sodium, hydrogen, carbon, and oxygen combine in the ratio of 1:1:1:3, respectively, for the formation of one molecule of baking soda.
These atoms give up their individual characteristics and together with other atoms acquire properties that are unique to baking soda.
It does not dissociate into its constituent elements through simple physical processes. All these are the features of a compound therefore, baking soda is a compound.
Sodium bicarbonate is an ionic compound in which the molecules break into separate ions viz. Na+ and HCO3- in an aqueous solution. Further dilution results in the formation of CO32- ions.
As the formation of ions is the property of compounds. Therefore, baking soda can be rightly called a compound.
Is Baking Soda a Mixture?
As per the definition mixtures are the substances formed by blending of two or more different types of
atoms or ions.
However, the mixtures are different from compounds as the different atoms or ions in a mixture is
neither chemically bonded nor they are present in a specific ratio.
The atoms still exhibit their individual properties. Also, the components of a mixture can be easily separated from one another by physical processes.
However, the different atoms chemically bond in definite proportions to form a molecule of baking soda. Also, the properties of baking soda are different from any of its fundamental elements.
Hence, baking soda is not a mixture.


Why Baking Soda Is Not a Mixture?
Scientifically, baking soda is considered a form of pure compound substance. Therefore, this
leavening agent is not considered a mixture, despite its combination of components because of its
homogenous properties.
This differs from what a heterogenous mixture possesses—since this composition shows every visible detail coming from different substances.
Sodium bicarbonate (general name, baking soda) is a by-product of sodium with hydrogen, oxygen, and carbon molecules. It results in a white crystal with a bitter aftertaste. When mixed with acid, it releases the alkalic binds—producing carbon dioxide. Carbon dioxide is essential for baking since it allows the bread to expand.
Aside from moisture, the air is essential to diffuse the complex combination of materials quickly. It produces a baked product that with a much robust flavor profile compared to its original structure.
If baking soda is not a mixture, what is it? The answer is baking soda is a form of pure homogenous compound substance. For those that have a minimal chemistry background, it can be simplified as:
- Baking soda is a pure substance, which means that it is made with natural elements and compounds that produce a homogenous appearance. As a result, baking soda is uniformly white with a crystalline gloss.
- Again, sodium bicarbonate is a compound produced by mixing sodium, hydrogen, carbon, and oxygen.
- It is a homogenous mixture, which means that it has a uniform shape and size. Baking & Food Preparation
Baking soda available in the grocery store is pure, food-grade sodium bicarbonate. Bakers add a small amount of baking soda to the mixture of flour, sugar, eggs, butter and other ingredients in cakes, cookies and other baked goods. The resulting chemical reaction then helps batter expand or rise inside a hot oven. Without baking soda and this chemical reaction, muffins, cakes and breads would fall flat.
Personal Care Products & Medicines
In skin care and personal care products like lotions and bath salts, sodium bicarbonate helps control a product’s acid-base balance to keep it from spoiling. In toothpaste, sodium bicarbonate helps to remove stains from teeth by dislodging tiny particles of food or beverages that can blemish tooth enamel. It is also a common ingredient in deodorant because it can help neutralize smelly, acidic scents.
It works by quickly neutralizing stomach acid and temporarily relieving symptoms of acid reflux.


baking soda vc baking powder
| bicarbonate of soda | Baking Powder |
| Mainly component is sodium Bicarbonate | Contains many components including Bicarbonates (typically baking soda), and acid salts. |
| Does not contain Monocalcium Phosphate | Contain Monocalcium Phosphate, which reacts with NaHCO3 |
| when wetted and heated. | |
| Reacts immediately with acids | It does not immediately react when exposed to acids. |
| Short leavening process | The leavening process extended with the help of a second acid. |
| when compared to baking powder products, due to shorter reaction duration. | Gives fluffier products from baking |
Sodium bicarbonate:
A bicarbonate (HCO3–) that’s bound with a sodium atom (related compounds use potassium or ammonium to similar effect).moreover, When added to water, the bicarbonate dissolves and is able to react with acids to generate CO2.
When it comes into contact with acids and water, it emits carbon dioxide, thereby extending doughs and batters to create baked goods with a porous surface. It is an inexpensive, non-toxic, and easy-to-handle ingredient that doesn’t offer an off taste in the finished product.
Baking Powder:
A self-contained leavening system that generates carbon dioxide in the presence of water. Baking powders by definition contain a baking soda and acids for that baking soda to react with.
The idea that these are categories, not single ingredients, is probably foreign to most home cooks, but the chemicals that make up a baking powder or baking soda can vary. Industrial food manufacturers use different compositions and particulate sizes depending upon the food being produced.
- Single-acting baking powders require only moisture to release gas. For all practical purposes, no single-acting baking powders are solid today. Practically single acting baking powders releases gas too quickly to be useful for most products.
- Double-acting baking powders releases some gas when cold but they require heat for complete reaction.
What Elements Make Up Sodium bicarbonate?
Sodium
Sodium is an alkali metal that bonds readily with other elements or ions. Alone, it is a soft but violent element that burns in the air and reacts violently with water. But when bonded with a bicarbonate ion (HCO3), it creates the harmless baking soda compound used by cooks worldwide.
Hydrogen
Hydrogen is the most common element in the universe and naturally occurs as an odorless, colorless, highly flammable gas. Moreover,It forms highly diverse compounds, from explosive rocket fuel and caustic acids to the weak base that absorbs refrigerator odors under the common name of baking soda.
Oxygen
Besides sustaining life as an integral part of the air you breathe and the water you drink, oxygen also forms the bicarbonate ion that creates baking soda. This ion makes baking soda a good pH regulator because it reacts with both acids and bases to create neutral salts. This characteristic makes baking soda effective for eliminating odors and for treating heartburn caused by excess stomach acid.

Jekyll and Hyde Compound
One of baking soda’s most common uses is for cooking, often as a leavening agent in baked goods. Chemical leavening requires an acidic catalyst in the batter, such as yogurt or buttermilk. On contact with the sodium bicarbonate, this causes the release of carbon dioxide in a simple acid-base reaction.3 Alternatively, baking soda can release smaller volumes of carbon dioxide without an acid simply via the process of thermal decomposition at temperatures above 50°C, although this typically leaves a characteristic bitter flavor. Either way, the release of gas into the mixture as it cooks changes a dish’s density and texture.
If ingested in different ways, the gas-producing property of baking soda (sodium bicarbonate) can cause very different effects. For people with acid reflux, sodium bicarbonate can act as an antacid to settle the stomach. It can also get rid of unwanted cockroaches, as feeding them a mix of bicarbonate and sugar behind the refrigerator can cause their internal organs to explode.
MORE POSTS:




