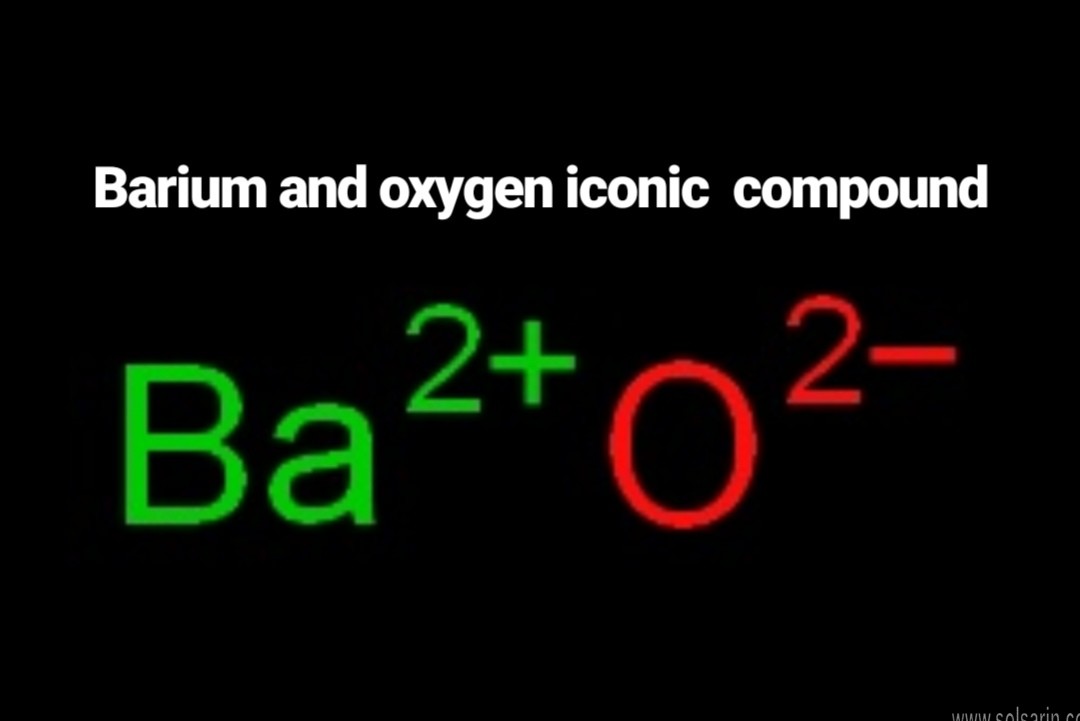barium and oxygen ionic compound
Hi, welcome to solsarin site, in this post we want to talk about“barium and oxygen ionic compound”,
thank you for choosing us.
barium and oxygen ionic compound
Barium oxide is an ionic compound , compromised of a barium metal cation, and an oxygen non-metal anion. Looking at the periodic table, oxygen is in group 6 or 16 , and will therefore have an oxidation number of −2 , while barium sits in group 2 , and will have an oxidation number of +2 .
What does oxygen and barium make?
Barium reacts with oxygen when exposed to air, forming a thin passivating layer of BaO on the surface. When ingnited, barium reacts with both oxygen and nitrogen, forming a mixture of BaO, Ba 3 N 2 and BaO 2. 2 Ba (s) + O 2 (g) 2 BaO (s).
Types of compound
Ionic compounds are compounds composed of ions, charged particles that form when an atom (or group of atoms, in the case of polyatomic ions) gains or loses electrons.
- A cation is a positively charged ion
- An anion is a negatively charged ion.
Covalent or molecular compounds form when elements share electrons in a covalent bond to form molecules. Molecular compounds are electrically neutral.
Ionic compounds are (usually) formed when a metal reacts with a nonmetal (or a polyatomic ion). Covalent compounds are formed when two nonmetals react with each other. Since hydrogen is a nonmetal, binary compounds containing hydrogen are also usually covalent compounds.
- Metal + Nonmetal —> ionic compound (usually)
- Metal + Polyatomic ion —> ionic compound (usually)
- Nonmetal + Nonmetal —> covalent compound (usually)
- Hydrogen + Nonmetal —> covalent compound (usually)

Ionic Compounds
When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily gain electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound.
For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas (group 17) accepts one electron to form a chloride anion, Cl−, the resulting compound, NaCl, is composed of sodium ions and chloride ions in the ratio of one Na+ ion for each Cl− ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form CaCl2, which is composed of Ca2+ and Cl− ions in the ratio of one Ca2+ ion to two Cl− ions.
A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. However, it is not always true (for example, aluminum chloride, AlCl3, is not ionic).
Ionic Compounds
You can often recognize ionic compounds because of their properties. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For example, sodium chloride melts at 801 °C and boils at 1413 °C. (As a comparison, the molecular compound water melts at 0 °C and boils at 100 °C.) In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). When molten, however, it can conduct electricity because its ions are able to move freely through the liquid.
In every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. Thus, ionic compounds are electrically neutral overall, even though they contain positive and negative ions. We can use this observation to help us write the formula of an ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.

Is barium oxide an ionic compound?
Yes. Barium oxide is an ionic compound. Generally a metal with a nonmetal will form an ionic compound.
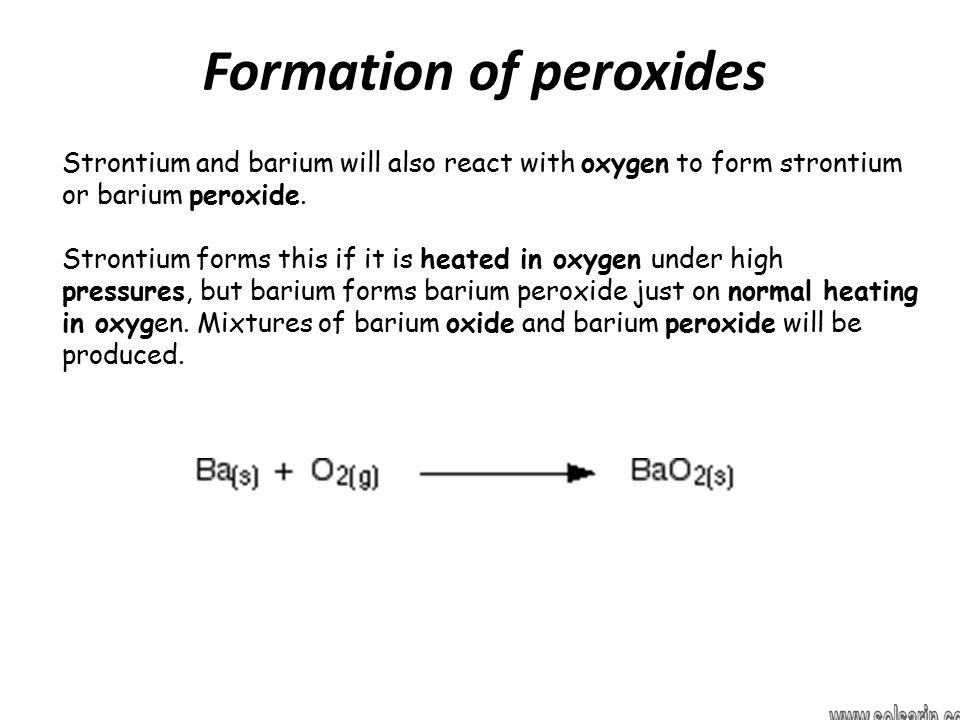
What type of oxide is BaO2?
Barium peroxide is the inorganic compound with the formula BaO2. This white solid (gray when impure) is one of the most common inorganic peroxides, and it was the first peroxide compound discovered.
Is barium oxide covalent or ionic?
Explanation: Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion.
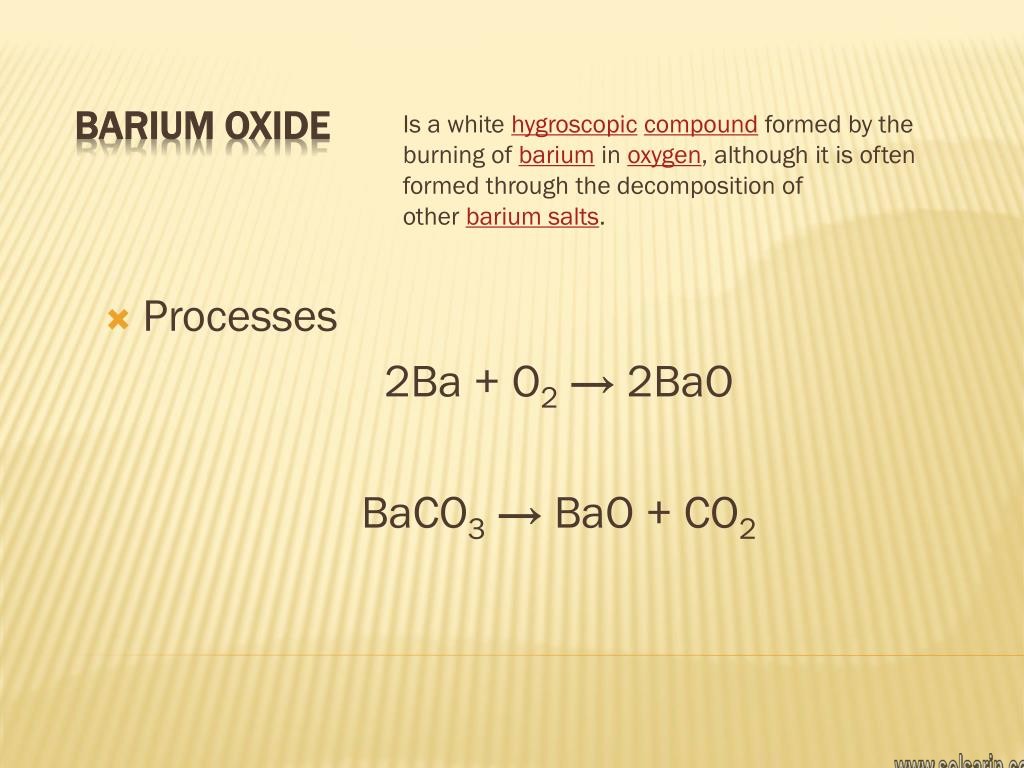
Barium Oxide Formula
Properties Of Barium Oxide
| Chemical formula | BaO |
| Molecular weight | 153.326 g/mol |
| Chemical Name | Calcined baryta, Baria, Barium monoxide, Barium protoxide |
| Density | 5.72 g/cm3 |
| Melting point | 1,923°C |
| Boiling Point | ~ 2,000°C |

Uses of barium oxide
We use it as a coating for hot cathodes that we use in cathode ray tubes. It replaces lead (II) oxide in the production of certain kinds of glass such as optical crown glass. Whereas lead oxide raises the refractive index and it also raises the dispersive power than barium oxide does not alter. In addition, we use it in the reaction of ethylene oxide and alcohol as an ethoxylation catalyst, which takes place between 150 and 200 oC.
Through heat fluctuation, it is also a source of pure oxygen. In addition, it freely oxidizes to BaO2 by the formation of peroxide ion. Furthermore, the complete peroxidation of BaO to BaO2 decomposes befalls at a moderate temperature but the increasing entropy of the O2 molecule at high temperatures means that BaO2 decomposes to O2 and BaO at 902 oC.
This method was earlier used to produce oxygen before the air separation became the dominant method at the beginning of the 20th century. This method is named the Brin process after its inventor.
The availability of barium oxide is in pellets, pieces, powder, sputtering targets, tablets, and nanopowder. Generally, it is instantly available in greatest volumes such as high purity, ultra-high purity, submicron, and nanopowder forms.
Barium oxide is used as a cathode for manufacturing cathode ray tubes, which were a TV-component in past. Barium is also used as catalyst for chemical reaction and can be used as a component in the production of some glasses.
Production of Barium
oxideBarium oxide or barium monoxide is prepared by heating barium carbonate(BaCO3). It may also be prepared by the thermal decomposition of the Barium Nitrate (Ba(NO3)2) compound. It is often prepared by the decomposition of other Barium salts. The chemical reactions for the generation of the compound barium oxide are as follows.2Ba + O2 → 2BaOBaCO3→ BaO + CO2In the above reaction, the barium carbonate (BaCO3) is heated and is decomposed to obtain Barium oxide, and during the reaction carbon dioxide is released.
Formulas of Ionic Compounds
For an ionic compound to be stable, the positive charges have to equal the negative charges. In NaCl, sodium (Na) is +1 and the chloride is 1. In MgO the magnesium is +2 and the oxygen (O) is 2 and so again the charges cancel each other out. Remember the charges come from the chart above.
Can we combine sodium (Na+) with oxygen (O2-)? Yes! To make a stable compound we need 2 sodiums for every one oxygen.
There is rule for finding the correct formula. In every ionic formula the cation is written first and the anion written second. In the formula, the charge on one becomes the subscripts of the other.

How are ionic compounds named?
What is the compound formula for barium and oxygen?
Is barium peroxide harmful for hair?
2nd dangerous ingredient present is heavy metal Barium in the form of Barium Peroxide is used in henna based hair dye (like in poor quality Black Henna) preparations as an oxidizing agent. Barium is a known poisonous ingredient & irritant to eyes/skin. … This action weakens & damages the hair cuticle to great extent.