how well does tungsten conduct electricity
Hello dear friends, thank you for choosing us. In this post on the solsarin site, we will talk about “how well does tungsten conduct electricity”.
Stay with us.
Thank you for your choice.


A metal consists of a lattice of atoms, each with an outer shell of electrons that freely dissociate from their parent atoms and travel through the lattice. This is also known as a positive ionic lattice.
This ‘sea’ of dissociable electrons allows the metal to conduct electric current. When an electrical potential difference (a voltage) is applied across the metal, the resulting electric field causes electrons to drift towards the positive terminal.
The actual drift velocity of electrons is very small, in the order of magnitude of a meter per hour. However, as the electrons are densely packed in the material, the electromagnetic field is propagated through the metal at the speed of light
What is tungsten?
Tungsten is referred to as a transitional metal because it is found in the middle of the periodic table. The color of tungsten ranges between steel-gray and whitish. What makes tungsten stand out is its exceptionally high melting point of 3,410 degrees Celsius.
It is the highest when compared to other metals on Earth. The other thing that makes tungsten unique is that even in extremely high temperatures, it can maintain its strength.
Tungsten is mostly used in the manufacturing of alloys. That purpose is to infuse the advantages that tungsten has to the metal it has been mixed with. It means that it can increase the strength, hardness, flexibility, and the tensile of steel.

SYMBOL
W
ATOMIC NUMBER
74
ATOMIC MASS
183.85
FAMILY
Group 6 (VIB)
Transition metal
PRONUNCIATION
TUNG-stun
Credit for the discovery of tungsten is often divided among three men—Spanish scientists Don Fausto D’Elhuyard (1755-1833) and his brother Don Juan Jose D’Elhuyard (1754-96), and Swedish chemist Carl Wilhelm Scheele (1742-86). Tungsten’s chemical symbol, W, is taken from an alternative name for the element, wolfram.
Discovery And Naming
The first mention of tungsten and its compounds can be traced to about 1761. German chemist Johann Gottlob Lehmann (1719-67) was studying a mineral known as wolframite. He found two new substances in the mineral but did not recognize that they were new elements.
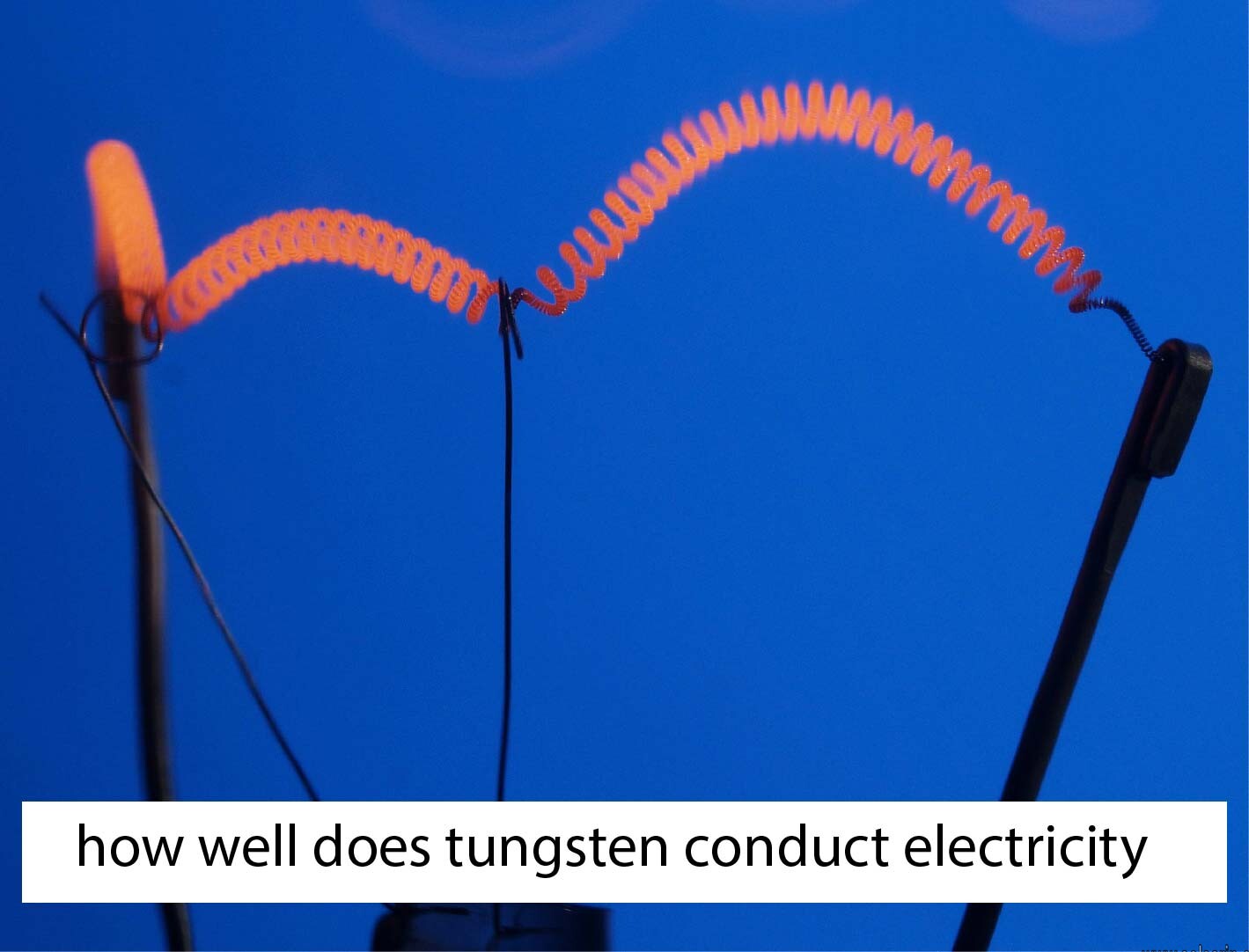
You will find that 90 percent of the tungsten alloys in the market get used in construction and mining, as well as in metalworking and electrical machinery. The other place that you find tungsten use is in incandescent lights. The thin wire that makes up the filament is made of tungsten. That’s because it will not melt even when the current heats the cable.
Tungsten is also ab inactive metal, meaning that it will not react with oxygen when it is at room temperature. It is also not corrosive, but it will corrode when exposed to a high temperature of 400 degrees Celsius. Generally, the metal will not create with acids, but it will dissolve in nitric acid or aqua regia.


More posts:
- what proof is smirnoff red white and berry vodka
- how do i level up my weapons on eternal
- finals has robert horry
- how much percentage salt in seawater
- what is pandora box movie about
Does tungsten conduct electricity? Why?
YES. The virtue that tungsten is metal it means that it does conduct electricity. Tungsten does conduct electricity currents quite well, such as when compared to iron and nickel. It is, however, not a good conductor as compared to other metals though it does get the job done. You will find that tungsten is used in manufacturing integrates circuits and other electronic components and parts.
Do Tungsten Carbide rings conduct electricity?
Before we get into the answer of whether tungsten carbide rings conduct electricity, we first need to state what it is. It is a chemical compound containing equal parts of carbon atoms and tungsten. In its basic form, it is a powder.
Tungsten carbide is almost twice as durable as steel and with double the density as well. It stands halfway between lead and gold. When it comes to finishing tungsten carbide rings, only things that are stronger such as diamond powder or wheels can polish and give the required finishing.
That truly speaks of the hardness of tungsten in general. When it comes to jewelry, tungsten is usually in the form of cemented carbide.
Tungsten carbide by itself has impressive qualities, as well. The compound is also able to withstand very high temperatures, and it is considered the strongest structural material. Additionally, tungsten carbide rings will not conduct electricity because they are ceramic instead of metallic.
That is why you will find that they are brittle and can crack under a hard blow. However, the impact needs to be extreme.


How to wear tungsten rings safely?
You ought not to be concerned about wearing tungsten rings. That is because all other materials such as platinum, titanium, and gold also conduct electricity. It is also unlikely that you would be coming into contact with the electric current at any one point. You can, therefore, wear your tungsten ring without worrying about it.
If you don’t want to have a ring that conducts electricity at all, then you can opt for the tungsten carbide. It is quite low conductivity compared to other metals.
The other aspect of safety comes in when we are talking about being able to have the ring removed during an emergency. There is a misconception that because of the hardness, it would be impossible to remove. However, it is even better than other metals such as gold and silver because of the fact it shatters instead of bending.
The medical team can use vice grips to crush the ring with care. In the case of other rings, it requires more time to cut through the metal; during an emergency, time is of the essence.
About twenty years later, Scheele also studied this mineral. He produced from it a white acidic powder. Scheele knew the powder was a new substance. But he could not isolate a pure element from it. Scheele’s discovery was actually tungstic acid (H2WO4). (See sidebar on Scheele in the chlorine entry in Volume 1.)
Tungsten metal was prepared for the first time in 1783 by the D’Elhuyard brothers. In 1777, they were sent to Sweden to study mineralogy. After their return to Spain, the brothers worked together on a number of projects. One project involved an analysis of wolframite. They produced tungstic acid like Scheele but went one step further. They found a way to obtain pure tungsten metal from the acid. For this work, they are generally given credit as the discoverers of tungsten.
The name tungsten is taken from the Swedish phrase that means “heavy stone.” In some parts of the world, the element is still called by another name, wolfram. This name comes from the German expression Wolf rahm, or “wolf froth (foam).” The element’s chemical symbol is taken from the German name rather than the Swedish name.
Physical Properties
Tungsten is a hard brittle solid whose color ranges from steel-gray to nearly white. Its melting point is the highest of any metal, 3,410°C (6,170°F) and its boiling point is about 5,900°C (10,600°F). Its density is about 19.3 grams per cubic centimeter. Tungsten conducts electrical current very well.
Chemical Properties
Tungsten is a relatively inactive metal. It does not combine with oxygen at room temperatures. It does corrode (rust) at temperatures above 400°C (700°F. also It does not react very readily with acids, although it does dissolve in nitric acid or aqua regia. Aqua regia is a mixture of hydrochloric and nitric acids. It often reacts with materials that do not react with either acid separately.
Occurrence In Nature
Tungsten never occurs as a free element in nature. Its most common ores are the minerals scheelite, or calcium tungstate (CaWO4) and wolframite, or iron manganese tungstate (Fe,MnWO4). The abundance of tungsten in the Earth’s crust is thought to be about 1.5 parts per million. It is one of the more rare elements.