Sulfur Barium Ionic Compound
Hey guys! We return with an amazing topic. It is about “Sulfur Barium Ionic Compound”. This topic will be placed in the General Information & Scientific category in solsarin‘s site. If you are interested in such topics follow us.


Barium sulfide
Barium sulfide is the inorganic compound with the formula BaS. BaS is the barium compound produced on the largest scale. It is an important precursor to other barium compounds including BaCO3 and the pigment, ZnS/BaSO4. Like other chalcogenides of the alkaline earth metals, BaS is a short wavelength emitter for electronic displays. It is colorless, although like many sulfides, it is commonly obtained in impure colored forms.
Discovery, production and properties
BaS was prepared by the Italian alchemist Vincentius (or Vincentinus) Casciarolus (or Casciorolus, 1571-1624) via the thermo-chemical reduction of BaSO4 (available as the mineral barite). It is currently manufactured by an improved version of Casciarolus’s process using coke in place of flour and charcoal. This kind of conversion is called a carbothermic reaction:
- BaSO4 + 2 C → BaS + 2 CO2
and also:
- BaSO4 + 4 C → BaS + 4 CO
The basic method remains in use today. BaS dissolves in water. These aqueous solutions, when treated with sodium carbonate or carbon dioxide, give a white solid of barium carbonate, a source material for many commercial barium compounds.
According to Harvey (1957), in 1603, Vincenzo Cascariolo used barite, found at the bottom of Mount Paterno near Bologna, in one of his non-fruitful attempts to produce gold. After grinding and heating the mineral with charcoal under reducing conditions, he obtained a persistent luminescent material rapidly called Lapis Boloniensis, or Bolognian stone. The phosphorescence of the material obtained by Casciarolo made it a curiosity.
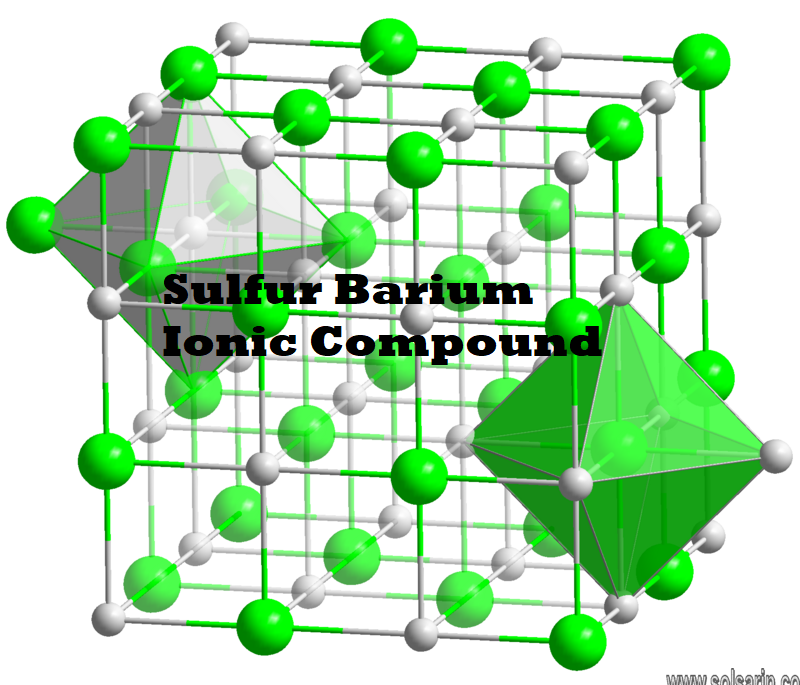
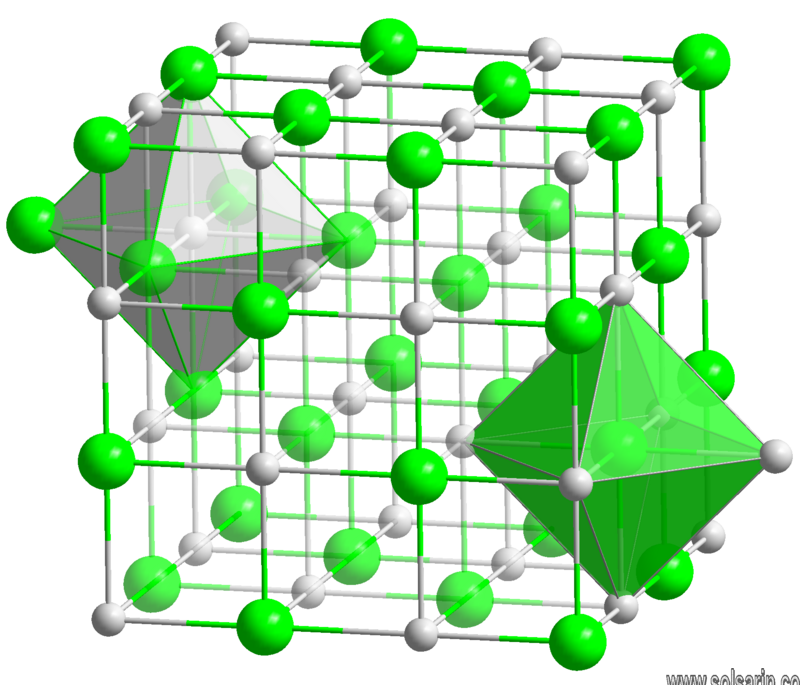
BaS crystallizes with the NaCl structure, featuring octahedral Ba2+ and S2− centres.
The observed melting point of barium sulfide is highly sensitive to impurities.
Formula and structure
The barium sulfide chemical formula is BaS. The molar mass is 169.39 g/mol. The molecule is formed by one barium cation Ba2+ and one sulfide anion S2-. The two ions are bound trough an ionic bond. The geometry of the molecule is octahedral with one cation surrounded by eight anions and vice versa. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence
It is not found in nature. Barium sulfide is produced as described below.
Preparation: Barium sulfide is prepared through the Casciarolus’ process using coke as a source of carbon and also using the cheap and largely available barium sulfate:
BaSO4 + 2 C → BaS + 2 CO2
Physical properties
Barium sulfide is a white solid. It is phosphorescent. The density of this salt is 4.25 g/mL. Its melting point is 2235 °C and above this temperature, it decomposes. Barium sulfide is slightly soluble in cold water but it is highly solubility in hot water. It is insoluble in ethanol and organic solvents.
Chemical properties
Barium sulfide exhibits the characteristic properties of other sulfides; it shows interesting behavior against the light. First, barium sulfides can deviate X-rays, so it is used in biomedical application to check tissues in details. It can also emit in short wavelength, so it is used in electronics equipments.
Uses
Barium sulfide is used as source for preparing other barium compounds such as the barium carbonate or barium sulfate. It is particularly used in the preparation of the pigment lithopone, which is a mixture of barium sulfate and zinc sulfide.
BaS + ZnSO4 → ZnS · BaSO4
To be used in the manufacturing of pigments, it should be in a pure form, due to some impurities can give color to the material. It is also used in the manufacturing of electronic devices for being a short wavelength emitter.
Health effects / safety hazards: Barium sulfide is extremely poisonous and can release toxic fumes. It is toxic for the aquatic life and environment. Barium sulfide is flammable and can react violently with water.
Group 17 (H, F, Cl, Br, I) Alkaline Earth Compounds
Barium Bromide
Barium bromide has the molecular formula of BaBr2. Like barium chloride, it is soluble in water but is toxic if ingested. BaBr2 crystallizes in a PbCl2 motif, as white orthorhombic crystals that are deliquescent. In aqueous solution, BaBr2 behaves as a simple salt. It adopts the same type of PbCl2 type of structures as BaCl2.

Cell Parameters
PbCl2 type, SG = 0P12: a = 4.935 Å, b = 8.252 Å, c = 9.915 Å, α = β = γ = 90°, cell volume = 402.77 Å3, cell occupancy is shown in Table 2.73.
TABLE 2.73.
| No | Site notation | Atom | Multiplicity | Wyckoff | Site symmetry | x | y | z | Occupancy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Br1 | Br | 4 | c | .m. | 0.0227 | 1/4 | 0.6621 | 1.0 |
| 2 | Br2 | Br | 4 | c | .m. | 0.1425 | 1/4 | 0.0745 | 1.0 |
| 3 | Ba1 | Ba | 4 | c | .m. | 0.2393 | 1/4 | 0.4045 | 1.0 |
PbCl2 type, SG = mP24: a = 4.896 Å, b = 9.302 Å, c = 12.773 Å, α = 92.6°, β = γ = 90°, cell volume = 581.36 Å3, cell occupancy is shown in Table 2.74.
TABLE 2.74.
| No | Site notation | Atom | Multiplicity | Wyckoff | Site symmetry | x | y | z | Occupancy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Br2 | Br | 4 | e | 1 | 0.07 | 0.227 | 0.072 | 1.0 |
| 2 | Ba1 | Ba | 4 | e | 1 | 0.12 | 0.27 | 0.334 | 1.0 |
| 3 | Br4 | Br | 4 | e | 1 | 0.165 | 0.727 | 0.24 | 1.0 |
| 4 | Ba2 | Ba | 4 | e | 1 | 0.38 | 0.273 | 0.13 | 1.0 |
| 5 | Br1 | Br | 4 | e | 1 | 0.455 | 0.273 | 0.355 | 1.0 |
| 6 | Br3 | Br | 4 | e | 1 | 0.71 | 0.227 | 0.03 | 1.0 |
Barium bromide reacts with the SO42− ion to produce insoluble BaSO4 as a precipitate:
Similar reactions occur with oxalic acid, HF and H3PO4. Barium bromide can be prepared from barium sulfide or barium carbonate by reaction with hydrobromic acid to give hydrated barium bromide:
Barium bromide can be crystallized from its solution as a dihydrate, BaBr2·2H2O (molecular weight: 333.19), which forms the anhydrate by heating to 120 °C. It dissolves in its own water-of-hydration at 75 °C before decomposing to the anhydrate. Barium bromide is a precursor to chemicals used in photography as well as to form other bromides.
Historically, barium bromide was used to purify radium in a process of fractional crystallization devised by Marie Curie. Since radium precipitates preferentially in a solution of barium bromide, the ratio of radium to barium in the precipitate was found to be higher than the ratio in the solution.
The solubility of barium bromide in water is shown in Table 2.75.
TABLE 2.75.
| g/100 ml of water | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Substance | Formula | 0 °C | 10 °C | 15 °C | 20 °C | 30 °C | 50 °C | 70 °C | 90 °C |
| Barium bromide | BaBr2 | 98 | 101 | 104 | 109 | 114 | 123 | 135 | 149 |
Physical properties are listed in Table 2.76.
TABLE 2.76.
| CAS number | 10553-31-8 (anhydrous) 7791-28-8 (dihydrate) |
| Molecular formula | BaBr2 (anhydrous) |
| Molecular weight | 297.14 g/mol |
| Density | 4.78 g/cm3 (anhydrous) |
| Melting point | 857 °C |
| Boiling point | 1835 °C |
| Crystal structure | Orthorhombic, oP12 Space group = Pnma, No. 62 |
| Standard enthalpy of formation | ΔfH |
lithopone
The lithopone, brilliant white pigment used in paints, inks, leather, paper, linoleum, and face powder. Lithopone was developed in the 1870s as a substitute or supplement for lead carbonate (white lead), to overcome its drawbacks of toxicity, poor weathering, and darkening in atmospheres that contain sulfur compounds. Lithopone is an insoluble mixture of barium sulfate and zinc sulfide that precipitates upon mixing solutions of barium sulfide and zinc sulfate.
The precipitate is recovered by filtration, then calcined (roasted) at temperatures above 600° C (1,112° F). Although it has been replaced in many applications by titanium dioxide, introduced after World War I, it is still widely used in a number of products, such as water paints.


carbon black
carbon black, any of a group of intensely black, finely divided forms of amorphous carbon, usually obtained as soot from partial combustion of hydrocarbons, used principally as reinforcing agents in automobile tires and other rubber products but also as extremely black pigments of high hiding power in printing ink, paint, and carbon paper.
This is also used in protective coatings, plastics, and resistors for electronic circuits. As a reinforcing filler it greatly increases resistance to wear and abrasion. About one fourth of the weight of a standard automobile tire is carbon black.
For tires on vehicles on which it is necessary to avoid building up an electrostatic charge, such as oil trucks and hospital operating carts, even more carbon black is added to make the rubber electrically conducting.
Carbon black particles are usually spherical in shape and less regularly crystalline than graphite. Carbon black changes into graphite if heated at 3,000° C (5,400° F) for a prolonged period. Among the most finely divided materials known, carbon blacks vary widely in particle size depending on the process by which they are made.
Channel or impingement black is made by the impingement of smoky flames from tiny jets on iron channels; the deposited black is scraped off by moving the channels over stationary scrapers. Furnace blacks are made in refractory chambers by incomplete combustion of any of various types of gaseous or liquid hydrocarbons. Thermal blacks are produced in the absence of air when hydrocarbons are decomposed by contact with heated refractories.
Lampblack, the oldest known black pigment, is produced by burning oil, usually coal-tar creosote, in shallow pans, in a furnace with the draft regulated to give a heavy smoke cloud. Acetylene black is produced in refractory chambers in the absence of air by the decomposition of acetylene gas preheated to 800° C (1,500° F). It is used in applications requiring high electrical conductivity, such as dry cells.