acetanilide polarity
Hi, welcome to solsarin site, in this post we want to talk about“acetanilide polarity”,
thank you for choosing us.
acetanilide polarity
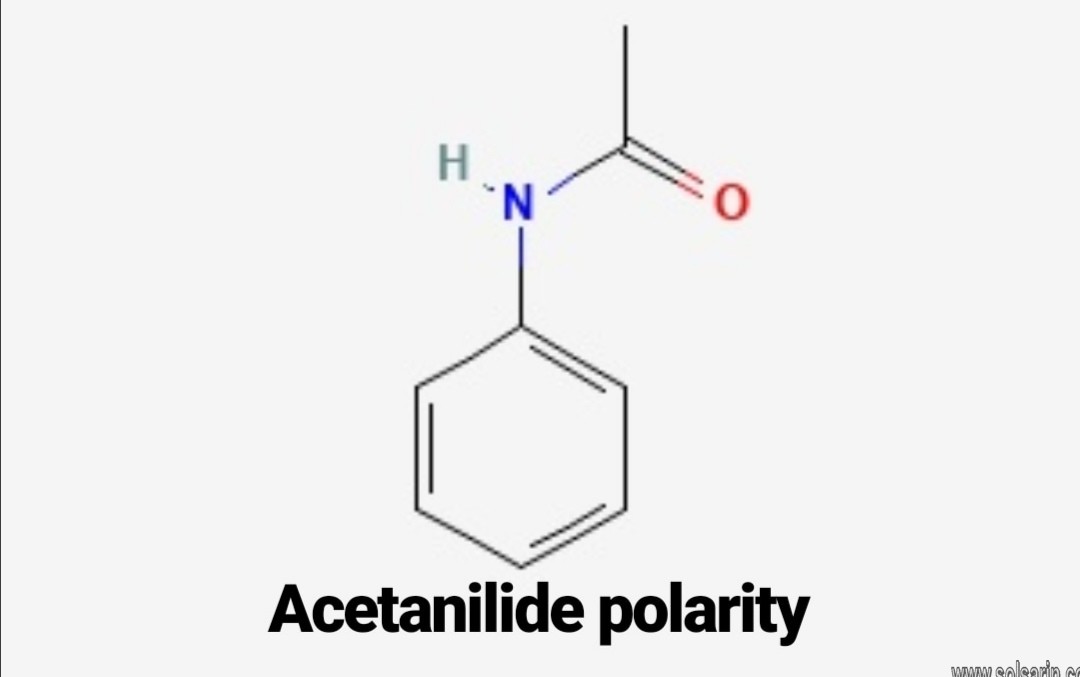
Acetanilide is the least polar out of these 5 derivatives of benzene. In acetanilide the benzene ring provides is responsible for its non-polarity. Even though it has polar groups like C-N, C=O. present, still accounts for low polarity.
How many polar bonds does Acetanilide have?
Chemical Structure Description The acetanilide molecule contains a total of 19 bond(s) There are 10 non-H bond(s), 7 multiple bond(s), 1 rotatable bond(s), 1 double bond(s), 6 aromatic bond(s), 1 six-membered ring(s) and 1 secondary amide(s) (aliphatic).
How can you determine the polarity of bonds?
The terms “polar” and “nonpolar” usually refer to covalent bonds. To determine the polarity of a covalent bond using numerical means, find the difference between the electronegativity of the atoms; if the result is between 0.4 and 1.7, then, generally, the bond is polar covalent.

Is Acetanilide Polar or Nonpolar?
What is polar and non-polar?
Polar
“In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other.
Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds.moreover, Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points.
Polar molecules
A polar molecule has a net dipole as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically.


How do you determine the polarity of a bond without electronegativity chart?
To review the steps:
- Draw the Lewis structure.
- Figure out the geometry (using VSEPR theory)
- Visualize or draw the geometry.
- Find the net dipole moment (you don’t have to actually do calculations if you can visualize it)
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
Is acetanilide an acid or base?
Acetanilide is a weak base with a pH value near 8. It is a weak base because of the resonance structures it shows. moreover,The nitrogen atom of the amide group does not act as a proton acceptor or a nucleophile.
What kind of bonds are found in acetanilide?
Acetanilide has four types of covalent bonds: multiple C-C bonds and C-H bonds; two C-N bonds; one N-H bond; and one C=O bond. According to the University of Wisconsin-Eau Claire, the C-C and C-H
bonds are considered nonpolar, while other bonds are considered polar. However, acetanilide is only slightly soluble in water.
What is the functional group of acetanilide?
The functional group present in acetanilide is the amide group, >CONH-. moreover, The nitrogen atom has two hydrogen atoms bonded to it in an amide.moreover, In acetanilide, one of the two hydrogen atoms gives way to a bond with a benzene ring.
Acetanilide
acetanilide, synthetic organic compound introduced in therapy in 1886 as a fever-reducing drugs. Its effectiveness in relieving pain was discovered soon thereafter, and it was used as an alternative to
aspirin for many years in treating such common complaints as headache, menstrual cramps, and rheumatism. Excessive or prolonged use engenders toxic side effects: it interferes with the function of hemoglobin, the oxygen-carrying pigment of the blood. In the body acetanilide is mostly converted to
acetaminophen, which has replaced acetanilide in therapy because it is less likely to induce blood disorders.
It’s called acetanilide and although it used to be a commonly prescribed medication for light pain
management, it quickly lost its allure among medical professionals after some serious toxicity
concerns began to arise. moreover,Today, we’re going to be talking about acetanilide in terms of its
chemical formula, resonance structures, and some of its important derivatives.

Structure & Chemical Formula
Acetanilide is an organic chemical compound (meaning it’s composed of carbon and hydrogen
mostly) that is classified as an amide in terms of its functional group. This means that it has the carbonyl group (carbon-oxygen double bond) bonded directly to a nitrogen atom.
Acetanilide only contains four types of atoms, which include carbon, hydrogen, nitrogen, and oxygen. For instance, the C6 H5 portion of the chemical formula represents the aromatic ring, and the NHCOCH3 piece represents the amide functional group.
A lot of times, it’s helpful to break molecules up into parts in order to simplify them. It’s kind of like taking what looks like a complex math problem. Although you may think it’s impossible to solve, once you break it down into simpler portions it begins to make more sense and is not so intimidating.
Main Difference – Aniline vs Acetanilide
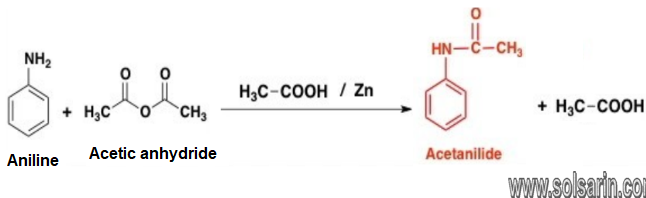
Aniline and acetanilide are nitrogen-containing organic compounds. moreover, They have very different chemical and physical properties. Aniline has many uses in different industries. Acetanilide is an aromatic amide. It is very weak base, and the basicity is even less than water. It is a solid compound at room temperature.however, The major production process of acetanilide involves the reaction between acetic anhydride and aniline. The main difference between aniline and acetanilide is that aniline is a yellowish brown oily liquid at room temperature whereas acetanilide is a white to gray solid compound.
What is Anilines?
Aniline is a type of organic base which is used in the making of several dyes, explosives, plastics,
drugs, and rubber, and photographic chemicals. Anilines are the organic compounds that lie in the
class of groups coming in the organic chemistry that is referred to as aminobenzene or phenylamine. These compounds are known to be toxic and to be one of the classes of the aromatic amines. moreover, They are used in a variety of industrial applications and possess all the characteristics of that of an aromatic compound.moreover, The aniline compounds are known to have the formula C6H5NH2 in which the amino group is attached to the phenyl group.
Anilines – Structure
Aniline, also known as aminobenzene or phenylamine, has 6 carbon (C) atoms, 7 hydrogen (H) atoms, and 1 nitrogen (N) atom in its chemical formula of C6H7N or C6H5NH2. Because aniline has an amino group in its structure, it is also an amine, hence it is classified as an aromatic amine.
Aniline is a musty, fishy-smelling yellowish to brownish, greasy liquid. -6°C melting point; 184°C boiling temperature; 158°F flash point Water-insoluble and somewhat denser than water. Vapors are more dense than air. Because aniline is an aromatic molecule that combines readily with other
aromatic compounds, a low aniline point suggests a low diesel index.moreover, A high aniline point suggests a highly paraffinic gasoline with a high Diesel index and excellent ignition quality.
The above image shows the structure of an Aniline compound. moreover, These compounds have the formula C6H5NH2 with a phenyl group (C6H5) attached to the amino group (NH2) as shown.
Aniline is in the form of a yellowish and somewhat brownish oily liquid having a musty and a fishy odour. It smells like the odour of a rotten fish. It is a kind of chemical substance which is a flammable liquid and has an unpleasant odour.The compound is soluble in water.
Aniline can also be colourless to light brown. It has a chemical formula of C6H5NH2 or C6H7N and since it has 6 carbon atoms, 1 nitrogen atom and 7 hydrogen atoms in its chemical formula, it is classified under organic compounds.


Is the word aniline polar or nonpolar?
I’ll tell you the polar or nonpolar list below. If you want to quickly find the word you want to search,
use Ctrl + F, then type the word you want to search.
How is the aniline point related to the volume of Anil?
The aniline point depends on the products V M (δ anil − δ oil) 2, were V M is the average molecular
volume, and δ anil and δ oil are the Hildebrand solubility parameters for aniline and for the oil.
The aniline point reflects the structure of base oils. Increasing molecular volumes raises the aniline point.
Are there any unprotonated molecules of aniline?
Under these conditions, there is practically no unprotonated aniline present and the substrate undergoing nitration is the anilinium ion. We get m -nitroaniline under these conditions. Nitrous acid
is certainly a weak enough acid so as to lead us to expect many unprotonated molecules of an aromatic amine in its presence.
MORE POSTS: